Sodium Nitrate Formula
Sodium nitrate formula is also known as nitrate of soda or cubic metre formula. It is a nitrate salt of sodium that is inorganic.
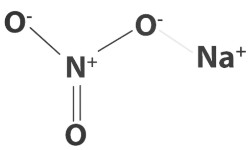
The sodium nitrate formula consists of sodium as a cation (Na⁺) and nitrate as an anion (NO³⁻).
The chemical representation of the sodium nitrate formula is NaNO₃.
The nitrogen atom bonds with three oxygen atoms via 2 single bonds and 1 single bond.
Characteristics of Sodium Nitrate
It is a white solid that is crystallised
It is odourless and has a slightly bitter taste
Highly soluble in water, ammonia, and hydrazine
It is non-combustible in nature
It is dissolvable in alcohol
Slightly dissolvable in pyridine
It is completely insoluble in acetone
The sodium nitrate is naturally bound to the mineral deposits that are found in Peru and Chile. These mineral deposits are known as the caliche ore.
Nitrates are accumulated on the land through precipitation of marine fog, gravitational settling of airborne KNO₃, Na₂SO₄, l, NaNO₃, and NaCl. Sea-spray oxidation or desiccation also accumulates nitrates on land.
Sodium nitrate was also known as ‘white gold’ during the 19th century. Wars raged over lands rich in sodium nitrate.
Uses of Sodium Nitrate
Sodium Nitrate is widely used in the manufacturing of
Pyrotechnics
Food preservatives
Glass
Fertilisers
Propellant
Smoke bombs
Solid rocket
Pottery enamels
Uses of Sodium Nitrate in the Food Industry
Sodium nitrate is used for meat curing in the food industry. It has no antioxidant activity but it becomes functional when it is reduced to nitrite. It is used for stabilisation of meat colour, improvement of texture, antimicrobial activity, elimination of warmed-over flavour, and the development of the characteristics of cured meat flavour.
Structural Representation of Sodium Nitrate
Properties of Sodium Nitrate
Chemical Formula- NaNO₃
Molecular weight- 84.9947 g/mol
Density- 2.257 g/cm³
Melting point- 308 °C
Boiling point- 380 °C
Risks Involved Around the Use of Sodium Nitrate
With the rise of sodium nitrate, there are increased chances of death due to diseases like Parkinson’s disease, diabetes, stomach cancer, and Alzheimer’s disease.
Solved Example
Question: Show the preparation of industrial sodium nitrate by conducting a neutralization reaction between nitric acid and sodium carbonate.
Answer: The following equation shows the preparation of industrial Sodium Nitrate:
NaHCO₃ + HNO₃ NaNO₃ + H₂O + CO₂
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out