Sodium Acetate Formula
Sodium Acetate is an anhydrous salt of sodium, and an alter to Acetic Acid. Sodium Acetate dissolves in water to form ions of sodium and ions of acetate.
Sodium acetate is a salt of ethanoic acid, also known as acetic acid, consisting of two atoms of carbon, three atoms of hydrogen, one atom of sodium, and two atoms of oxygen.
The molecular formula and the chemical formula of Sodium Acetate are cited below:
The molecular formula of Sodium Acetate: C2H3NaO2
The chemical formula of Sodium acetate: CH3COONa
The one important thing to remember is that the molecular formula and the chemical formula of Sodium Acetate is one of the ordinary or common chemical compounds.
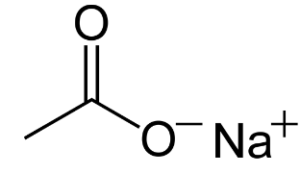
The structure of NaOAc is depicted below:
The structural formula of Sodium Ethanoate or Sodium Acetate
It should be observed that Sodium Acetate or sodium Ethanoate takes place naturally in the tissues of animals and plants. Not only that but it is used majorly in food industries as a seasoning agent or substance. It can also be cooked or prepared by the process of neutralisation of acetic acid.
Application of Sodium Acetate
Sodium Acetate has several applications that are mentioned below:
Biotechnological
Sodium Acetate or Sodium Ethanoate is used as a source of carbon for culturing bacteria. Sodium Acetate is also used to increase yields or production of DNA separation or isolation by ethanol precipitation.
Industrial
Sodium acetate is also used in textile industries to neutralise sulphuric acid waste streams and is also used as a photoresist while utilising aniline dyes. In the intermediate process of making cotton or disposable pads of cotton, Sodium Acetate is used to get rid of the build-up of dynamic or static electricity.
Food
Sodium Acetate is used as a seasoning agent in food industries, sometimes in the contour of sodium diacetate. It is also used to give vinegar and salt flavour to potato chips or is sometimes used as a substitute for vinegar for potato chips because it doesn’t add moisture to the outcome.
Buffer Solution
An elementary salt of Acetic Acid, Sodium acetate, can react as a buffer to continue a relatively constant level of pH. This is a useful application used in biochemical applications where the reactions are dependent on pH value.
Solved Examples
1. Is Sodium Acetate a water-soluble compound?
Sodium Acetate is a water-soluble compound, as it dissolves with water efficiency. If a temperature rise occurs, the solubility of sodium acetate also rises in water.
2. What is the reason behind Sodium Acetate generating heat?
Sodium acetate generates heat because when it is heated over 58 degrees Celsius, the solidified sodium acetate trihydrate loses its capacity for hydration and disintegrates in the resultant steam.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




