
Achieve your IIT dreams with Unacademy
“Coulomb’s law says that the force of attraction/repulsion between two electric charges is directly proportional to their magnitudes and inversely proportional to their distance”.
Coulomb’s law operates along the line connecting the two charged bodie’s centres. The law is also known as Coulomb’s inverse-square law because it describes an inverse square relationship between force and distance between two charged entities. Coulomb’s Law is regarded as an electrical equivalent of Newton’s Universal Law of Gravitation since the relation it derives is quite similar to the gravitational force acting between two large masses.
Body
The quantitative expression for the effect of these three variables on electric force is called Coulomb’s law. Coulomb’s law states that the energy between two charged objects is directly proportional to each object’s charge and inversely proportional to the separation distance between them. This can be expressed mathematically as:
F= k * Q1* Q2/ d2
In order to calculate the force exerted by Q2 on the charge Q1, the formula that can be used are :-
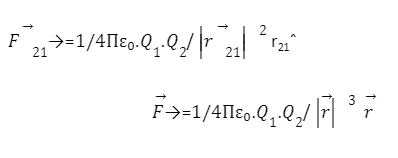
Questions on coulomb’s Law
Determine the electrostatic force between the two charges of magnitude 2 C and -1 C separated by a distance 4m in air.
Solution:
The first charge, q1 is +2 C.
The second charge, q2 is -1C.
The distance between the two charges is 4 m.
The formula to calculate electrostatic force between the charges is:
F = k q1q2 / r2
F = (9 × 109 N m2/ C2)(+2 C)(-1 C) / (4 m2)
=4.5 × 109 N
The distance between the two electrons in contact is equal to 1Å. Determine the Coulomb force between them.
Solution:
The charge on an electron is -1.6 × 10-19 C.
The distance between the two charges is 1 Å.
The formula to calculate electrostatic force between the two electrons is:
F = k (q2 / r2)
F = (9 × 109 Nm2/ C2) [(-1.6 × 10-19 ) C2 / (1 Å]2
= 2.3 × 10−8 N
5) Consider a system of two charges of magnitude 2 × 10-7C and 4.5 × 10-7C which is acted upon by a force of 0.1 N. What is the distance between the two charges?
The first charge, q1 is 2 × 10-7C.
The second charge, q2 is 4.5 × 10-7C.
The force acted upon them, F is 0.1 N.
The formula to calculate electrostatic force between the charges is:
F = k q1q2 / r2
0.1 N = (9 × 109 Nm2/ C2)(2 × 10-7C)(4.5 × 10-7C) / (r)2
r = 0.09 m
Hence, the distance between the two charges, r is 0.9 m.
5) Determine the magnitude of the two identical charges, when the electrostatic force between these two identical charges is 1000 N and are separated by a distance of 0.1 m.
Solution:
The distance between the two charges is 0.1 m.
The force acted upon them, F is 1000 N.
The formula to calculate electrostatic force between the charges is:
F = k q2 / r2
where q is the charge.
Rearrange the above formula for q as,
q2 = Fr2 / k
q2 = (1000 N) (0.1)2m2 / (9 × 109Nm2/ C2)
q = 0.33 × 10-5 C
so,the magnitude of the charge is 0.33 × 10-5 C.
6) placed at a distance from each other such that the force of F N acts between these two charges. If 60% of the charge from one is transferred to another. Determine how much the value of force changes in this case.
Solution:
Initially, the electrostatic force between the two charges is given by,
F = k q2 / r2 ……(1)
Now, when the charge is transferred, the electrostatic force becomes,
F’ = k q1q2 / r2 ……(2)
The transferred charge is,
60 % of q = 60 / 100 × q = 3 / 5 q
Therefore, charge q1 = q – 3 / 5 q
= 2 / 5 q
And the charge q2 = q + 3 / 5 q
= 8 / 5 q
So, net force between these charges is,
F’ = k q1 q2/ r2
= k (2 / 5 q) (8 / 5 q) / r2
= 16 / 25 F
7) A specific charge Q is split into two components, r, and Q-r. What is the relation between Q and q if the two portions are separated by d and have the greatest Coulomb repulsion?
Solution:
Q is divided into charges Q-r and r separated by a distance d.
We know,
F= k * Q1* Q2/ d2
F = k r (Q-r) / d2
Now, to maximize this force
d F / d q = 0
(Q-r) – r = 0
2 r =Q
r= Q / 2
Conclusion
The electric force is inversely proportional to the square of Coulomb’s law. Furthermore, this law is used in deriving Gauss’ law accurately for general cases. Charges at rest exert the following properties according to Coulomb’s law- where like charges repel one another and unlike charges attract each other. The vector form of Coulomb’s law provides the direction of electric fields caused by charges. Two negative charges repel one another, while a positive charge attracts a negative charge. In physics, charges act in accordance with their lines of attraction.
Explore IIT JEE Coaching in Different Cities
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




