Iron oxide Formula
Haemoglobin, a type of protein found in red blood cells that transports oxygen from the lungs to all areas of the body, is made up primarily of iron.
One of the most common types of chemical compounds, including other components of oxygen, is iron oxide.
Iron Oxide
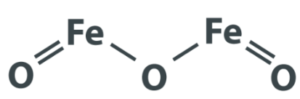
The chemical formula for iron (III) oxide is Fe2O3When iron oxidizes, it produces this substance. The process of oxidation is when an atom loses an electron. Iron (III) forms an ionic connection with oxygen in this process.
Two iron atoms and three oxygen atoms make up iron (III) oxide. The oxidation states must be evaluated in order to derive the chemical formula for iron (III) oxide. When forming ionic bonding, most atoms lose a certain amount of electrons. During bonding, iron is unique in that it can lose a varied amount of electrons. Iron becomes oxidized when it loses electrons.
There are multiple possible oxidation states for iron, but the two most frequent are Fe(II), which has an oxidation state of 2, and Fe(III), which has an oxidation state of 3.
The diagram can be shown as:
Iron oxides are common natural chemicals that are very simple to make in the lab. Oxides, hydroxides, and oxide-hydroxides are among the 16 types of iron oxides.
These minerals form as a result of water reactions at different redox and pH levels.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



