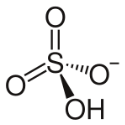
Hydrogen Sulfate Formula
Small Description: Bisulfate, often known as hydrogen sulphate, is a sulfuric acid salt having the chemical formula HSO4–. Hydrogen sulphates or bisulfates are chemical compounds containing this ion.
A hydrogen sulfate is also called sulphur oxoanion. It’s a sulfuric acid conjugate base. It is a sulphate conjugate acid. This is also acidic in nature. They can be used as a milder acid alternative to sulfuric acid. It’s a sulfuric acid salt. Sulfuric acid is deprotonated once in these compounds. It is deprotonated twice in the sulphate ion.
Structure of Hydrogen Sulphate
Properties of Hydrogen Sulphate
| Name of Chemical | Hydrogen Sulfate |
| Also Known as | Bisulfate |
| Molecular Formula | HSO4– |
| Molar Mass | 97.0715 g/mol |
| Density | 2.345 g/cm3 |
| Water Solubility | Yes |
| Melting Point | 58.5 °C |
Chemical reaction of Hydrogen Sulfate
Sulfate and hydronium ions are formed when hydrogen sulphate reacts with distilled water. Its chemical formula is as follows:
Uses of Hydrogen Sulfate – HSO4–
Hydrogen Sulfate – HSO4– is used to remove residual chlorine following wastewater treatment as an alternative for liquid sulphur dioxide. Because it was previously manufactured through an outmoded procedure, sodium salt of hydrogen sulphate is popularly referred to as niter cake.
The scarlet fluid in the resin soothes the skin, and when used in styptic pencils, it stops the flow of blood.
Solved Examples
How do you calculate the S oxidation number in hydrogen sulphate?
The formula for hydrogen sulfate is HSO4–. According to the general rule of oxidation number, all the oxidation numbers must add up to the ion’s charge.
H has an oxidation number of one.
O has an oxidation number of -2.
So, according to the question, 1 + S + (-2)x4 = 6, which means Sulfur’s oxidation number in hydrogen sulphate is 6.
What charge do hydrogen sulfate molecules have?
The net charge stored by hydrogen sulfate molecules is -1 in magnitude. Sulphuric acid, with the chemical formula H2SO4, is formed when this molecule gains a proton.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out