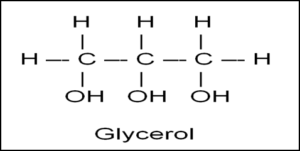
Glycerol Formula
Glycerol is a polyol compound with the chemical formula C3H8O3. It is colourless, its taste is sweet, and it’s not toxic. It is used as a bacterial culture medium. Glycerol backbones are found in lipids which are known as glycerides.
About the Topic
The IUPAC name of glycerol is Propane-1,2,3-triol. There are some other glycerol names: Glycerin, Glycerine, Propanetriol, 1,2,3-Trihydroxypropane, 1,2,3-Propanetriol.
The molar mass of glycerol is 92.094 g·mol−1. The density is 1.261 g/cm3, and the appearance of glycerol is a Colourless hygroscopic liquid. Glycerol is odourless.
The melting point of glycerol is 17.8 °C, and the Boiling point is 290 °C. Glycerol is miscible soluble in water. It’s in an achiral form but prochiral because of the reactions of one or two alcohols. The density of glycerol is 1.261 g/ml.
C3H8O3 has three carbon (C) atoms, eight hydrogens (H) atoms, and three oxygen (O) atoms. The pool is the term that is also used to define glycerol as it comes from hydroxyl groups which are OH groups. Glycerol is soluble in water because of hydroxyl, derived from the OH group. These groups are also attached to the carbon atoms.
Glycerol is mainly obtained from different plants and animals. When in a plant or animal, a chain of carboxylic acids has triglycerides and esters. Triglycerides of hydrolysis, saponification, or transesterification form the glycerol in plants and animals. Triglycerides combine with sodium hydroxide to produce glycerol. There is another type of glycerol: synthetic glycerol that can be derived from propene.
Glycerol is mainly used in Personal Care Products, pharmaceuticals, and the food industry. Pharmaceutical companies mainly use glycerol to make tablets smoother and easier to solve because sometimes tablets are very hard, and we can’t swallow them easily. It is also used in syrups, mainly cough syrup or any other syrup in which glycerol is added to prevent our throat from getting infected by the cough syrup.
In our daily life, the products we use like moisturiser, cream, face wash, and soap, and in all these skin care products, glycerol is used to make the products not harmful to our skin. In toothpaste, glycerol is also added to prevent our teeth from getting tooth disease. It is also added to the shampoo, conditioner, and other hair care products.
Glycerol is also used in the food industry to add some smoothness, shininess, and sweetness to the food products. Usage of glycerol is mainly found in the food industry.
Formula
C3H8O3
Solved Examples
- From what glycerol is formed?
Heating fats can form glycerol.
- What are the two main properties of glycerol?
There are various important properties of glycerol, but two of them which are important are colourless and hygroscopic.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




