Citric Acid:
Citric acid, having the molecular formula C6H8O7, is a powerful organic compound but a weak organic acid.
Citric acid comes in two different forms: monohydrate and water-free (anhydrous). Citric acid can be found in a variety of plants and fruits, including lemons and oranges, with the tomato having the highest concentration. It’s also known as tribasic acid.
Citric acid is a white crystalline substance with no odor and a sour taste. Its crystal structure is monoclinic.
Molecular Formula -C6H8O7
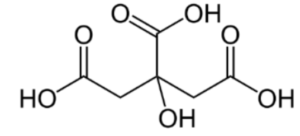
The extended formula is CH2COOH-C(OH)COOH-CH2COOH.
Its molar mass is 192.12 g mol-1.
IUPAC name = 2-hydroxypropane-1, 2, 3-tricarboxylic acid
Structure of Citric Acid:
Citric Acid’s Applications
- Citric acid is commonly found in soft drinks, juices, and other liquids. It imparts a tangy, citrus flavor to them. Citric acid is a common flavoring ingredient.
- It works as a food preservative because its acidic pH prevents the growth of many bacteria in food, preventing spoilage. Citric acid lowers the pH of frozen foods, rendering oxidative enzymes inactive.
- Citric acid is also used to soften the basic pH of soaps and detergents.
- It’s also utilized as a preservative in many cosmetics to keep the acid-base pH balance. It’s used in cosmetics to exfoliate dead skin, as well as to reduce wrinkles and even out skin tone.
- Citric acid is used as an acidulant to manage pH levels and works as an anticoagulant by chelating calcium in the blood.
- Lemon peels, a source of citric acid, are utilized to boost bone health in conditions like osteoporosis because they contain a high level of calcium and vitamin C, which aid in bone maintenance.
- In the pharmaceutical sector, citric acid is utilized as a preservative for keeping blood.
- Some ice businesses employ citric acid as an emulsifier to avoid using fat globules as an emulsifying agent.
- In caramel, citric acid is used to prevent sugar crystallization.
Physical properties:
- Citric acid is a crystalline white substance with no odor.
- It has a density of 1.542 g ml-1 (monohydrate form) and 1.665 g ml-1 (anhydrous form), with melting and boiling temperatures of 156 °C. and 310 °C respectively.
- Water, ether, acetone, ethanol, and methanol are extremely soluble, while toluene, benzene, and dichloromethane are insoluble.
Chemical Properties:
- Citric acid is a weak acid, it is always in equilibrium in aqueous solution between the anionic -1, -2, and -3 forms and the neutral form.
- The more negative the charge on the anion, the more acidic it will be, with the pH of the associated aqueous solution ranging from pH 2 (for the CH2COOH-C(OH)COOH-CH2COO–) to pH 8 (for the CH2 COO-C(OH)COO-CH2COO-³).
- Citric acid is employed as a pH buffer regulator in many industrial processes because of this. Another essential feature of citric acid is its ability to bind metals, generating chelate metals that are comparable to EDTA complexes.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




