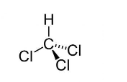
Chloroform Formula
Chloroform with the formula CHCl3 is a colourless organic compound. It is also used as a precursor to various refrigerants. Chloroform is a very powerful anaesthetic, euphoriant, and sedative when it gets inhaled by someone.
About the Topic
Chloroform has a very strong smell and is used as a precursor to PTFE. There are many other names for chloroform that scientists have given, like Methane, trichloride, Freon 20, Refrigerant-20, R-20, UN 1888, Methyl trichloride, Methenyl, trichloride, Methenyl chloride, and TCM.
IUPAC’s name for chloroform is Trichloromethane. In the laboratory, chloroform is prepared by heating the ethyl alcohol and later adding bleaching powder into it and some water to prepare chloroform. Later it has to go through a purification process. In the purification process, impure chloroform is washed with dilute caustic soda, and later it gets dried over anhydrous calcium chloride.
Chloroform is mainly used as a solvent that helps other substances dissolve. It is used in different industries like the paper and board industries. In the 1990s, Doctors used it for pain reduction during medical operations.
The appearance of chloroform is colourless and has a molar mass of 119.37 g·mol-1. While we smell it, chloroform gives us a smell of Misleadingly pleasant ethereal odour, leading to olfactory fatigue.
The melting point of chloroform is −63.5 °C, and the Boiling point is 61.15 °C. In Benzene, chloroform gets soluble quickly, and in diethyl ether, oils, ligroin, alcohol, CCl4, and CS2, it gets Miscible. The structure is in Molecular shape as Tetrahedral, and the Dipole moment is 1.15 D.
It’s said that 90% of chloroform comes from nature, and it mainly comes from the environment, which is approximately 66,0000 tonnes every year. The abiotic process is also a process of chloroform production in which it produces chloroform from the soil. Chloroform is not directly bioaccumulation in aquatic organisms.
Formula
CHCl3
Solved Examples:
1.What is the nature of chloroform?
Chloroform is a colourless organic compound. It’s a hard acid and has a pleasant, nonirritating odour. Melting Point is -63.5 °C, and Boiling Point is 61.2 °C. It has a slightly sweet taste. Another name for chloroform is Trichloromethane.
2.Which chemical is used in chloroform?
In the preparation of chloroform, the mainly used substance is chlorodifluoromethane (HCFC-22).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out