Chlorine Gas Formula:
Chlorine gas has the chemical structure Cl2 and has a molecular weight of 70 g/mol. Cl-Cl, commonly known as the element form of chlorine, has a structure that consists of two chlorine atoms connected by a covalent connection. Both atoms have an sp³ orientation, which means they have a tetrahedral structure. In the typical representations for organic compounds, its chemical structure can be expressed as follows.
![]() Preparation Methods of Chlorine
Preparation Methods of Chlorine
1. Process of Electrolysis
The gas can be obtained by electrolyzing salt water in a Nelson cell. This is the cheapest method and produces the purest form of the gas.
2. The Deacon’s Procedure
We can make the gas by oxidizing hydrochloric acid in the presence of cuprous chloride at 723K and 1 atmospheric pressure in this process.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




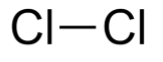 Preparation Methods of Chlorine
Preparation Methods of Chlorine