Calcium Phosphate Formula
Calcium phosphate has the chemical formula Ca3(PO4)2 and is a calcium salt of phosphoric acid. Calcium phosphate tribasic, or Tricalcium Phosphate, is another name for it.
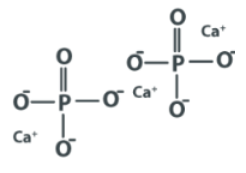
The formula comes from the fact that calcium phosphate is made up of two phosphorus atoms, three calcium atoms, and eight oxygen atoms.
Calcium phosphate contains the ions Ca2+ calcium ion and phosphate ion. Calcium phosphate is also found when the calcium ion is combined with orthophosphate, pyrophosphate, or metaphosphate ions. As a result, the chemical formulas for monocalcium phosphate, Ca(H2PO4)2, dicalcium phosphate, Ca2(H2PO4)2, and tricalcium phosphate, Ca3(H2PO4)2,.
Properties of Calcium phosphate
- Calcium phosphate melts at 1670 degrees Celsius
- When heated, it decomposes
- 310.18 is the molar mass of calcium phosphate
- Water doesn’t dissolve it
- Calcium phosphate is a white powder that has a powdery appearance
Physical properties
- Calcium phosphate is an odorless white powder.
- This salt has a density of 2.22 g/mL.
- It melts at 109 degrees Celsius and decomposes at 203 degrees Celsius.
- It is only marginally soluble in water, with a solubility of 2 g/100 mL.
- Inorganic solvents do not dissolve it.
Chemical Properties
Calcium phosphate is a stable and versatile chemical that can be used in a variety of reactions. As a result, it can be utilized in a variety of household items to react with other chemical substances. A leavening agent made from calcium phosphate and sodium bicarbonate is one of these instances.
Calcium phosphate structural formula
Solved Examples:
Is Calcium Phosphate Water Soluble?
Calcium is necessary for a variety of bodily activities, including bone production and maintenance. Calcium can also attach to other minerals (such as phosphate) and aid in their elimination from the body. Calcium phosphate is used to prevent and treat calcium deficiency.
How is calcium phosphate fertilizer made?
Mixing a fine calcium-containing material with diluted phosphoric acid containing at least 30% by weight of H3PO4to create calcium phosphate fertilizer, calcium silicate, and diluted phosphoric acid is a method of producing calcium phosphate fertilizer from a calcium-containing material that is reactive with diluted phosphoric acid to create calcium phosphate fertilizer, calcium silicate, and diluted phosphoric acid.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out