Alkanes:
Alkanes are hydrocarbons with only one connection between them. Because they contain hydrogen in every available place, they are known as saturated hydrocarbons. As a result, they have the general formula Cn H2n+2.
where,
- n is the number of carbons in a particular alkane.
Physical properties of alkanes:
- Non-polar chemicals are alkanes. Because the difference in electronegativities between Carbon and Hydrogen is nearly non-existent, they have almost no polarity.
- The force, on the other hand, grows greater as the molecules grow larger. As a result, the boiling and melting temperatures of more complex alkanes are greater.
- In their natural states, they can exist as solids, liquids, or gasses. In their native condition, unbranched alkanes are generally gasses. Methane, ethane, and other gasses are examples.
- Due to the weak van der Waal forces, they are also fully insoluble in water.
- They are, nevertheless, soluble in organic substances. Alkane van der Waal forces break here, and younger van der Waal forces take their place.
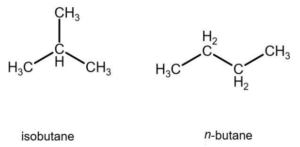
Butane
n-Butane (C4H10) is a colorless gas that is very soluble in water, unlike the first three alkanes.
Petroleum and liquefied natural gas are the primary basic materials used in its manufacturing. At low concentrations, it generates an explosive and flammable mixture with air. Its primary industrial use is as a raw material in the manufacture of butadiene and acetic acid.
It’s also utilized as a household fuel, a component of gasoline, a solvent, and a refrigerant.
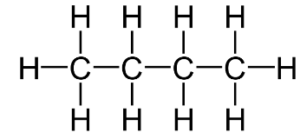
The bonds in butane can be shown as:
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out