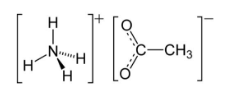
Ammonium Acetate Formula
Ammonium acetate is a chemical compound with the formula NH4CH3CO2. Ammonia and acetic acid make it a white hygroscopic solid. The melting point of ammonium acetate is
113 °C. It is soluble.
About the Topic
The molar mass of Ammonium acetate is 77.083 g·mol-1. The appearance of Ammonium acetate is White solid crystals and deliquescent. The density of Ammonium acetate is 1.17 g.cm³.
It smells slightly acetic. Other names for ammonium acetate are Mindererus, Ammonium ethanoate, and Azanium acetate. It is also a biodegradable agent and helps as a protein precipitating reagent in dialysis to remove all the contaminants via diffusion. PH level of ammonium acetate varies from pH 4.75 to pH 9.25.
Ammonium acetate is prepared when Glacial Acetic acid and ammonia react. Acetic acid with Ammonium Carbonate has to be neutralised to get ammonium acetate. Ammonium acetate is made from 2 carbon atoms, 7 Hydrogen atoms, 1 Nitrogen atom, and 2 Oxygen atoms. The IUPAC name of Ammonium Acetate is Ammonium Ethanoate.
2CH3COOH + (NH4)2CO3→ 2 CH3COONH4 + H2CO3
H2CO3→ CO2+ H2O
CH3COOH + NH3→ CH3COONH4
Ammonium acetate is mainly used for acetic acid to create a buffer because of the low pressure of Ammonium acetate, which is used to remove non-volatile salts while preparing samples for mass spectrometry. It is also used as a food additive that works as an acidity regulator in the food industry. It is also used in the preparation or synthesis of Acetamide. Also used as pesticides, herbicides, and non-steroidal anti-inflammatory drugs.
Formula
NH4CH3CO2
Solved Examples
- How ammonium acetate is a precursor of Acetamide?
NH4CH3CO2 → CH3C(O)NH2 + H2O
2. What happens when ammonium acetate is heated?
When ammonium acetate is heated, it gets converted from Ammonium to acetonitrile.
3.Is Ammonium acetate dangerous?
Higher exposure or inhalation of Ammonium may affect your health, like irritation in the mouth or body. The face is a very sensitive part of the body, so if Ammonium is directly heating our face or skin, it will affect our skin and cause red rashes. It may also affect our stomach if we inhale it in large quantities.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out