Aluminum Phosphate Formula
- Aluminum phosphate is formed by the anion PO4-3 (phosphate) and anion Al3+. The molecular or chemical formula of Aluminum phosphate is AlPO4. Aluminum phosphate occurs as a white crystalline powder.
Aluminum Phosphate Formula:
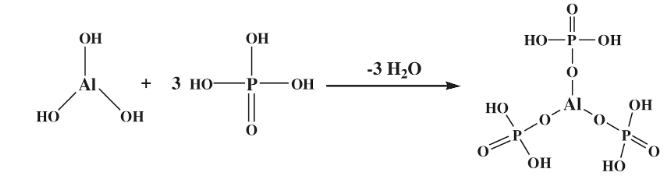
Hydroxyl groups of the Aluminum hydroxy phosphate are replaced by phosphate groups to from aluminum phosphate.AlPO4. The chemical reaction is as follows;
In its aqueous form, it is a colorless liquid it is insoluble in water melting point -1800°C
AlPO4 is prepared by exposing soluble aluminum salts to alkaline conditions contains hydrated aluminum orthophosphate with the formula AlPO4.
Aluminum phosphate dihydrate has a group of phosphate anions, aluminum cations, and water. It contains one phosphorus atom, 4 oxygen atoms, (3 shared with single bond and one with double bond), and one aluminum atom, generally it appears as dehydrate means aluminum phosphate along with 5 water ions.
Uses of Aluminum Phosphate:
- Aluminum phosphate is an odorless, white crystalline solid which is often used in liquid or get form.
- It is used in ceramics, dental cements, cosmetics, paints, paper and pharmaceuticals.
- Aluminum phosphate is made in ultrapure form by reaction between phosphoric acid and aluminum alkoxide.
- Aluminum phosphate formula, also known as Aluminum monophosphate AlPO4.
Aluminum phosphate is one of the most familiar chemical compound. It is naturally found in the form of a mineral named Berlinite. Many synthetic forms of aluminum phosphate are known.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




