What is the valency of lead?
The valencies of lead are +2, and +4 as it follows the concept of varying valency.
Lead is the group-14 metals element in the periodic table, with the symbol Pb and atomic number 82. In each of its six orbits, this element has 82 electrons which are placed in distinct orbits according to certain principles and order. Electron configuration is the arrangement of electrons in distinct orbits and orbitals of an atom in a specific order. Thus, for Pb electrons are distributed as 2, 8, 18, 32, 18, and 4 in electron shells K, L, M, N, O, and P respectively.
The following is an electronic configuration for a lead:
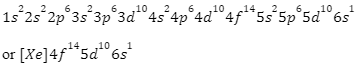
A number of valence electrons is defined as the number of electrons in an atom or molecule’s outer shell. In the case of lead, the 6th or outer shell contains four electrons. As a result, lead has four valence electrons.
Valency refers to the number of electrons that are gained or lost by an atom in order to complete its outermost shell. By completing their outer shell, or octet (8 electrons in the outermost shell)., the atoms become stable.
Now we know the lead has four electrons in its valence shell, thus we would assume it has a valency of 4 but lead follows the concept of varying valencies in different compounds which means an element shows distinct valency under different chemical reaction conditions. For example, lead has a valency of +2 in lead (ii) oxide (PbO), but a valency of +4 in lead dioxide (PbO2).
Lead as metal is used in car batteries, pigments, cable sheathing, lifting weights, ammunition, diving weight belts, lead crystal glass, radiation protection, and some soldiers. It’s also utilised in stained glass windows, for roofs in architecture and to store corrosive liquids.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






