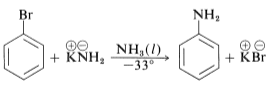When a nucleophile displaces a leaving group on an aromatic ring, the presence of an electron-withdrawing group increases the rate of nucleophilic aromatic substitution.
The nucleophilic aromatic substitution does not follow the SN2 reaction pathway due to the steric barrier of the benzene ring. When a noble leaving group is present, the SN1 reaction mechanism is activated, resulting in the removal of the leaving group and forming an aryl carbocation.
The elimination addition or addition elimination pathway, which is the substitution at the trivalent carbon atom that has been found to undergo sp3 hybridisation, is followed by the predominantly nucleophilic aromatic substitution.
Nucleophilic Aromatic Substitution Conditions
The electron-retreating institution is needed to activate the aromatic ring for nucleophilic aromatic substitution. Consequently, nucleophilic aromatic substituents are mainly determined within the nitro group.
When the electron-taking flight group leaves the ortho or parafunction, the resonance stabilisation of the negative rate inside the transition state is supported. Depending on electronegativity, excess of halogen atoms SN1 and SN2, nucleophilic aromatic substituents like -OH, -OR, -NH2, -SR, NH3 and other amines are commonly seen.
Nucleophilic Aromatic Substitution through Benzyne: Elimination-Addition Mechanism
Mechanisms for nucleophilic aromatic substitution have been proposed, one of which uses benzyne as the intermediate and is known as the benzyne mechanism.
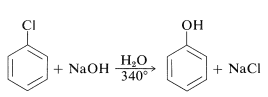
Aryl halides, a part of halobenzene, show low reactivity to nucleophilic reagents. This is mainly due to displacement with alkyl halides and active aryl halides. Substitutions do occur when high temperatures or solid bases are present. Here chlorobenzene reacts with sodium hydroxide, which requires a temperature of around 340 °C. This is part of a significant reaction.
In addition, the conjugate bases of the amines are converted to aryl chlorides, and bromides and iodides can be converted to the area mines ArNH2. Furthermore, at temperatures below 33 °C, the reaction of potassium amide with bromobenzene is relatively rapid.
Displacement reactions for the displacement of activated aryl halides are already regularly rearranged. In other words, the coming into the organisation no longer constantly occupies the same ring function because of the halogen substituent.
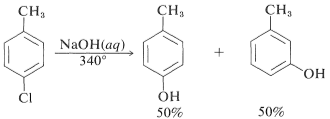
When 4-chloromethyl benzene is hydrolysed at 340 °, an equimolar mixture of 3- and 4-methyl benzene is produced
The sole production of 3-methyl benzenamine in the amination of 2-chloromethyl benzene is much more dramatic. This conclusion violates the notion of the least amount of structural change.
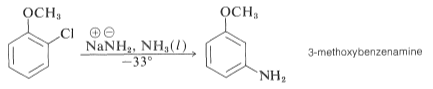
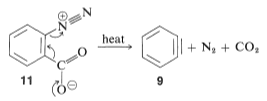
The elimination reaction produces benzyne, or dehydrobenzene, a highly reactive intermediate 9 that differs from benzene by having two fewer hydrogens and an additional link between two ortho carbons. Benzyne reacts quickly with any accessible nucleophile, such as ammonia, to produce a different product
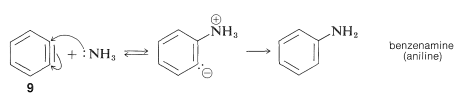
This step-by-step process involves the removal of base-catalysed hydrogen halide (HX) from the halide, as described below for bromobenzene amination
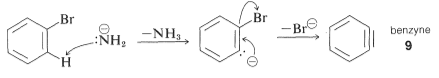
In these reactions, the nucleophile reacts with one or both carbons of the additional bond in the intermediate, causing rearrangements. Based on the symmetry of benzene, its rearrangement cannot be identified. The substitution of isomeric compounds can be benzene. Due to the interaction of hydroxide ion with 4-chloro-1-methylbenzene, 4-methylbenzene, 10 yields both 3- and 4-methylbenzene
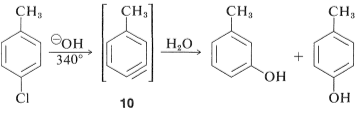
The base that paperwork the benzyne within the removal step is generated from the nucleophile inside the addition section in the initial benzyne reactions. Relying on the reaction conditions, this can now not always be the case.
Conclusion
Aryl halides are less reactive toward nucleophilic reagents due to displacement with alkyl halides and active aryl halides. Substitutions do occur when high temperatures or solid bases are present. For example, chlorobenzene reacts with sodium hydroxide, which requires a temperature of around 340 °C. The success with which the aryne may be created by one reagent but collected by another is critical to the synthetic value of aryne reactions.Another way to produce aryne is by thermally decomposing 1,2-disubstituted arene compound such as 11, in which both substituents are leaving groups – one leaving with an electron pair, the other leaving without
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out