Rearrangement is a process where an atom migrates to another atom within a molecule. The pinacol-pinacolone rearrangement is a type of molecular rearrangement where the atoms move to an electron-deficient carbon. It is used for the conversion of cyclic diols to spirocyclic ketones. This rearrangement is also used to produce many spirocyclic compounds. The 1, 2-migration in the pinacol-pinacolone rearrangement takes place under acyl conditions. This rearrangement is a technique for changing a 1, 2-diol over to a carbonyl compound.
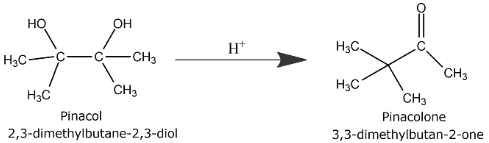
Pinacol and Pinacolone
Pinacols are compounds with 1,2-diols, i.e., they have 2 hydroxyl group gatherings. Every hydroxyl is connected to the section of a carbon particle.
Pinacolone is an inorganic compound. It has no colour and its odour is like camphor or peppermint. The name of pinacolone is three-dimethyl-two-butanone.
The pinacol-pinacolone rearrangement continues through an intermediate substance with a charge. In the rearrangement, 1,2-migration continuously takes place under acidic conditions. It is used to prepare ketones by dehydrating alcohol.
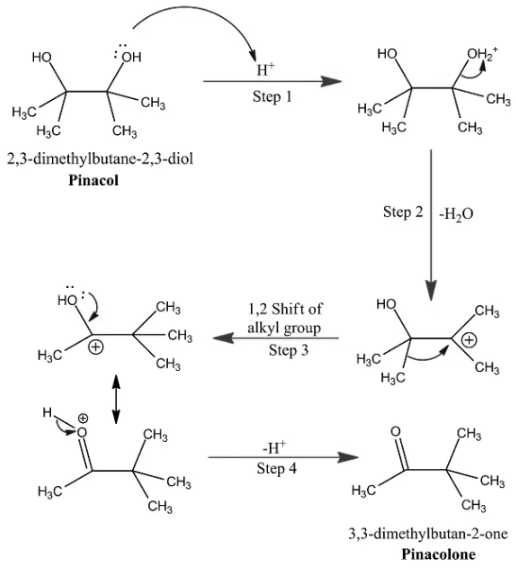
Rearrangement Mechanism
This type of rearrangement is an organic reaction characterised by removing water or alcohol. Concentrated sulphuric acid and heat are used to turn the rearrangement into 1,2-diols.
At 1st, the hydroxide is protonated within the existence of carbonation with the elimination of water from that. The tertiary carbocation is stronger and steady. The following step is the change of the alkyl group, which gets shifted to the positive carbon once the nucleon is eliminated by water and a carbonyl compound is formed.
It is an alcohol, and it undergoes rearrangement into an organic compound. This reaction follows the reaction method of SN1, for it ends up forming a radical from a glycol. The 2,4-DNP is again checked and is employed for confirmation of pinacolone.
The rearrangement helps to observe the presence of aldehydes and ketones in organic compounds. Once so many drops of the chemical agent have been mixed into the product, it forms an organic precipitate that indicates the presence of the radical.
Step-By-Step Reaction
Here is the step-by-step process of the reaction:
Step 1: Protonation of Cemical Group
The dihydric alcohol compound passes through acidic conditions. During this progression, a nucleon or chemical element particle H+ is about free from the acid. H2SO4 is unlinked to produce an H+ ion and SO42- ion. Consequently, the ion is connected to the chemical group already in pinacol. During this process, the hydroxide cluster of pinacol is protonated.
Step 2: Loss of a Water Molecule
A carbocation is made during this process. The smallest particles of water get separated from the compound and produce a carbocation. This carbocation is of a tertiary kind, and the next product is steady.
Step 3: Migration of methyl
The methyl shifts from one atom toward another charged atom of the carbocation.
Step 4: Deprotonation
The protonation in step 1 is headed towards deprotonation. The carbon-oxygen double bond, linked to a pinacol, withdraws from it.
Application of the Pinacol-Pinacolone Rearrangement
The pinacol-pinacolone reaction produces many complex compounds which are otherwise difficult to prepare. As such, it has a variety of applications, such as:
- Synthesis of pesticides
- Retrosynthetic analysis of vibunazole
- Creation of pinacidil and naminidil
- Production of ketones
- Synthesis of stilbestrol pinacolone
- Synthesis of carbonyl compounds from alkenes
- Production of triadimefon, an antimycotic agent
Conclusion
The pinacol-pinacolone rearrangement is an intermolecular reaction. It is one of the oldest rearrangements in history, discovered by German chemist Wilhelm Fitting in 1860. The pinacol-pinacolone rearrangement is used in the preparation of many carbonyl compounds. Thus, it is widely used in the pharmaceutical industry and the creation of pesticides and fungicides. It has significant value in the field of organic chemistry.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






