The electrophilic and nucleophilic aromatic substitution reactions are the elemental reactions in organic and inorganic chemistry.
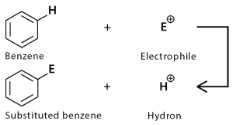
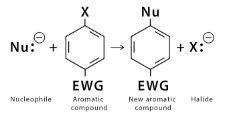
Electrophilic Aromatic Substitution is the reaction in which an electrophile substitutes hydrogen in the aromatic ring. In contrast, Nucleophilic Aromatic Substitution is the reaction in which a nucleophile substitutes a leaving group in the aromatic ring.
Electrophiles are electron-deficient species in a chemical reaction that readily accept electrons from electron-rich species to form a bonding. These can be Lewis Acid and Bronsted Acid.
For example, halogen molecules like Fluorine (F₂) and Chlorine (Cl₂), acids like hydronium ion (H₃O+), etc.
Nucleophiles are electron-rich species searching for a positive centre, the nucleus of an atom, to form bonding in a chemical reaction. Examples of nucleophiles are halogen anions (–, Cl–), etc., cyanide ion (CN–), ammonia(NH3)
Differentiation between Electrophilic and Nucleophilic Aromatic Substitution Reaction
Substitutes
Electrophilic Aromatic Substitution – Here, an electrophile attacks the aromatic ring. The electrophile being electron deficit seeks for the electron cloud in aromatic compounds, which forms an electron pool with C-C pi– electrons.
Nucleophilic Aromatic Substitution: Here, a nucleophile attacks the aromatic ring. The nucleophile has a positive centre and hence gets bonded with C. In this atom, there is a leaving group as the C–atom has a relative negative charge due to the delocalisation of electrons because of the electron-withdrawing groups present in the ring.
Aromatic Compound
Electrophilic Aromatic Substitution- The aromatic compound is nucleophilic, as it is electron-rich.
C6H6, a stable benzene ring with a C–C pi(π) electron cloud, acts as a nucleophilic, tending to attract the positive centre of the electrophile, which has fewer electrons.
Nucleophilic Aromatic Substitution- The aromatic compound is electron-deficient, hence electrophilic.
C6H2(NO2)3 is the aromatic compound with three electron-withdrawing groups and hence is an electron-deficient compound that tends to attract electron-rich nucleophiles.
Leaving Group
Electrophilic Aromatic Substitution: The leaving group is H+. The electrophilic aromatic substitution reaction mechanism includes the deprotonation of the tetrahedral carbon. Hence H+ is the leaving group that leaves the ring.
Nucleophilic Aromatic Substitution: The leaving group is not H+. It can be anything like Chlorine. Here, the leaving group is halides, mostly Fluorine, Chlorine, Bromine, etc. The C–X bond breaks and X (halide) leaves the ring.
Place of Attack
Electrophilic Aromatic Substitution– The place of binding of electrophile depends on the ortho-, meta- or para- positions that constitute the steric and electronic factors. The steric hindrance plays a role in the stability and arrangement of the atoms and molecules of the compound.
Nucleophilic Aromatic Substitution: The place of substitution depends on the position of the leaving group. The ortho-, meta- or para- position though not directly govern the place of attack, but the rate of Reaction is greatly affected by the position of the leaving and electron-withdrawing groups. For example, the nucleophilic aromatic Substitution reaction form-nitrophenyl fluoride is slower than p-nitrophenyl fluoride.
Mechanism
Electrophilic Aromatic Substitution
Step 1: Bonding of electrophile by the pi(π) electrons.
Here, the electrophile, an electron-rich species, attacks the pi(π) bond aromatic ring (benzene ring, C6H6 ). The pi (π) bond acts as a nucleophile here for the electrophile and attracts it. The delocalisation of the electron due to the attachment of the electrophile induces a positive charge to the attached carbon atom.
The electron from the pi(π) bond forms a bond with the electrophile, while the aromaticity of the compound is lost, forming a positively charged intermediate with a carbocation.
The electron-rich electrophilic substituents stabilise the electron-poor intermediate formed.
Step 2: The tetrahedral carbon deprotonates
From the intermediate formed in the first step, the C–H bond breaks, and thus the H+ goes off from the ring, and the aromaticity of the ring is restored, forming the C–C pi(π) bond.
Hence the electrophilic aromatic substitution product C6H5E is formed.
Nucleophilic Aromatic Substitution
Step 1: Attack the nucleophile on the electron-deficient aromatic compound.
The attachment of the nucleophile compound to the aromatic compound breaks the ring’s aromaticity. The delocalisation of the electron due to the attachment of the nucleophile induces a negative charge to the attached carbon atom. Hence, it forms a negatively charged intermediate. This is a rate-limiting phase.
The electron-withdrawing group (NO2) stabilises the electron-rich intermediate.
Step 2: The leaving group is expelled from the ring.
The negatively charged intermediate now expels the leaving group (for example, F2) from the ring. Hence the aromaticity of the ring is regained, and a nucleophilic aromatic substitution product is formed.
Hence, the reagent is an electrophile for electrophilic aromatic substitution reaction, whereas it uses a nucleophilic reagent for nucleophilic aromatic substitution reaction.
The intermediate form is positively charged in the electrophilic aromatic substitution reaction, whereas the intermediate is negatively charged for the nucleophilic aromatic substitution reaction.
Deprotonation occurs in the electrophilic aromatic substitution reaction, whereas the case is not the same for the nucleophilic aromatic substitution reaction.
The Rate of Reaction is Increased by
For electrophilic aromatic substitution reaction, a catalyst is mostly a Lewis acid, such as AlCl3 or FeCl3.
For nucleophilic aromatic substitution reaction, the reaction rate increases with the number of electron-withdrawing groups (NO2) present in the aromatic compound.
Conclusion
The electrophilic aromatic substitution and the nucleophilic aromatic substitution reactions exhibit a similar substitution, except that these reactions go in reverse polarity. Apart from the important differences, these share some common aspects, such as in both the reactions, electron sharing is observed.
To add to that, covalent bonds are formed for both of these reactions and along with the production of leaving groups (H+, F–), there is also the displacement of different groups in the substrate compound.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out