Kolbe electrolysis, also known as the Kolbe reaction, is an organic reaction named after Hermann Kolbe. (1) Formally, the Kolbe reaction is a decarboxylase dimerization of two carboxylic acids (or carboxylate ions). Overall, reaction is
When a mixture of two different carboxylates is used, all of their combinations are generally regarded as the organic product structures:
R1—R1 + R1—R2 + R2—R2 + 6 CO2 + 6 e → 3 R1COO + 3 R2COO R1—R1 + R1—R2 + R2—
R2 + 6 CO2 + 6 e
A two-stage radical process is involved in the reaction mechanism: electrochemical decarboxylation produces a radical intermediate, which combines to form a covalent bond.
(2)Electrolysis of acetic acid, for example, produces ethane and carbon dioxide:
CH3COOH → CH3COO− → CH3COO* → CH3* + CO. 2CH3 → CH3CH.
Another case in point is the production of 2,7-dimethyl-2,7-dinitrooctane from 4-methyl-4-nitrovaleric acid.
Mechanism of the Kolbe Reaction
The Kolbe reaction is a chemical process that involves carboxylation. When sodium phenoxide is allowed to absorb carbon dioxide, the resultant product is heated to 125 degrees Celsius and at a pressure of over a hundred atmospheres. Now an unstable intermediate has emerged.
This unstable intermediate undergoes a proton shift, which results in the creation of sodium salicylate. This mixture is now being treated with sulphuric acid. The salicylic acid is produced by acidifying the mixture. The following is an instance of the Kolbe reaction mechanism:
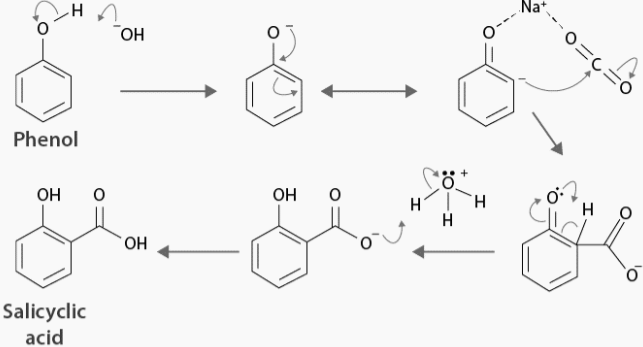
As a result of the Kolbe reaction, the necessary aromatic hydroxy acid – salicylic acid is created. It is possible to see a nucleophilic addition of sodium phenoxide.to carbon dioxide gas to form the salicylate in the mechanism.
Kolbe Reaction Applications
4-Hydroxybenzoic acid may be obtained by using Potassium Hydroxide in the Kolbe process. This is a necessary precursor for parabens (parahydroxybenzoate or ester of para-hydroxyl benzoic acid, used as a biocide in cosmetic products).
The Kolbe reaction may also be used to make 3-hydroxy-2-naphthoic acid, which is a common precursor of azo dyes and pigments.
By reacting salicylic acid with acetic anhydride, it is possible to produce aspirin. Aspirin is a popular pain reliever.
Preparation Of Alkane By Kolbe’s Electrolysis Method
Kolbe’s electrolysis method is a general method of preparation of substituted hydrocarbons from the substituted carboxylic acids by the use of the electric discharge method where carbon dioxide gas is released. It is one of the most widely used methods for the preparation of alkanes and substituted hydrocarbons.
The Kolbe reaction is a radical reaction that is essentially a decarboxylative dimerisation. An aqueous solution of sodium or potassium salt of carboxylic acid is electrolyzed in this reaction, resulting in the dissociation of the salt into carboxylate ion and sodium or potassium ions.
For the production of ethane and higher alkanes, this process is used.
Kolbe reaction:
CH3COOH(aq) + * ↔ CH3COO* + (H+ + e- ) (x2) ………….(1)
CH3COO* ↔ TSCH3-COO* ↔ CH3* + CO2(g) (x2) ………..(2)
2CH3* ↔ TSCH3-CH3* ↔ C2H6(g) + 2*……… (3)
Oxygen evolution reaction (OER):
H2O(aq) + * ↔ H2O* …………..(4)
H2O* ↔ OH* + (H+ + e- ) ……………(5)
OH* ↔ O* + (H+ + e- ) ………….(6)
O* + H2O(aq) ↔ OOH* + (H+ + e- )……….. (7)
OOH* ↔ O2(g) + (H+ + e- ) + * ……….. (8)
Where * denotes the surface sites for adsorption. In addition to the thermodynamics of the elementary steps, we have computed explicitly TSCH3-COO* and TSCH3-CH3*, the transition states corresponding to the decarboxylation of CH3COO* and dimerization of CH3* respectively, which interact significantly with the interfacial electric field and are decisive in the reaction energetics, as we discuss below. We have, for simplicity, neglected non-Kolbe processes in this analysis.
Conclusion
Kolbe electrolysis has been presented as a useful electrooxidation method for producing (un)symmetrical dimers from biomass-derived carboxylic acids. The reaction mechanism of Kolbe electrolysis, however, is still debated. We use a DFT-based microkinetic model to investigate the reaction mechanism of Kolbe electrolysis of acetic acid (CH3COOH) on pristine and partially oxidised anodes in this paper. We show that the shift in the rate-determining phase of the oxygen evolution process (OER) on the surface from OH* production to H2O adsorption causes the enormous Tafel slopes, or inflection zones, observed in tests on Pt anodes at high anodic potentials. In the presence of Kolbe species (i.e., CH3COO* and CH3*), activity passivation as a result of the inflection zone is worsened.
The activity of the Kolbe reaction during the inflection zone is determined by the CH3COO* decarboxylation and CH3* dimerization stages, according to our simulations. has no activity towards Kolbe products, in contrast to the surface, since the CH3COO* The decarboxylation step is limited throughout the potential range. The origin of the inflection zone and the identity of the rate limiting step are two main disputes in mechanistic investigations of Kolbe electrolysis on Pt anodes.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




