The science of the link between heat, work, and the properties of substances is known as thermodynamics. Because heat and work are inextricably linked by energy, thermodynamics is defined as the study of energy. Since energy is such an important and necessary component of our lives, we all need to understand thermodynamics. Furthermore, in order to effectively practise engineering, engineers and those aspiring to be engineers must know the fundamentals as well as applications of thermodynamics.
The Thermodynamic Laws are a summation and essence of human interactions with nature. While the Zeroth Law establishes the foundation for temperature measurement, the First and Second Laws specify the two qualities of energy and entropy, as well as the conservation and degradation of energy.
Zeroth Law of Thermodynamics
When a body ‘A’ is in thermal equilibrium with another body ‘b’ and with a body ‘C’ separately, then bodies ‘B’ and ‘C’ are likewise in thermal equilibrium with each other. The zeroth law of thermodynamics is defined by this statement. The law is based on the measurement of temperature.
The zeroth law of thermodynamics indicates that temperature is a significant measurement since it predicts whether or not heat will move between objects. Regardless of how the items interact, this is true. Heat can travel between two things even if they are not physically touching, according to the radiation mechanism of heat transmission. The zeroth law of thermodynamics states that no heat flow will occur if the systems are in thermal equilibrium.
Applications of Zeroth Law of Thermodynamics
The law is significant for the mathematical formulation of thermodynamics, or, to put it another way, for defining the mathematical definition of temperature. The most common application of this concept is to compare the temperatures of different things.
If we wish to accurately measure temperature, we’ll need a reference body and a property of that body that changes with temperature. The change in that attribute could be interpreted as a temperature change. The chosen attribute is referred to as a thermodynamic property.
Thermometers are the most prevalent application of the zeroth law of thermodynamics. Using a common thermometer with mercury in a tube, we may watch the zeroth law in operation. Just because the area of the tube remains constant as the temperature rises, the mercury expands. The height has increased as a result of this development. Now, the variation in the height of the mercury label indicates temperature changes and, in fact, serves us in measuring it.
First Law of Thermodynamics
The total energy of an isolated system is constant, according to the first law of thermodynamics. Energy can be changed into several forms, but it cannot be created or destroyed.
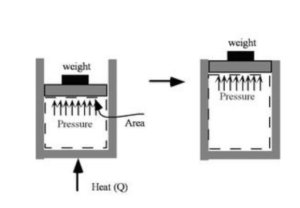
According to this law, some of the heat supplied to the system is utilised to change the internal energy, while the remainder is employed by the system to perform work. Mathematically,
ΔQ=ΔU+ΔW
ΔQ = Heat supplied
ΔW= Work done
ΔU = Change in the internal energy
There is a net heat transfer into the system if Q is positive, and there is work done by the system if W is positive. As a result, positive Q gives energy to the system whereas positive W depletes it.
Applications of First Law of Thermodynamics
- Isothermal Process: During an isothermal operation, the temperature of an ideal gas remains constant. This implies that the system’s heat is being used to operate against the environment. So,
ΔQ=ΔU+ΔW
ΔQ=W
ΔQ = Heat supplied
ΔW= Work done
ΔU = Change in the internal energy
- Melting Process: The internal energy of a solid increases when it melts into a liquid. Let m be the liquid’s mass and L be the solid’s latent heat. dQ = mL is the amount of heat absorbed by the system.
Let small expansion occurs, ∆V=0
dW=P∆V=0
Therefore,
ΔQ=ΔU+ΔW
ΔU=mL
As a result, during the melting process, internal energy increases.
- Heat Engines: The most common practical application of the First Law is the heat engine. Heat engines convert thermal energy to mechanical energy and vice versa. Open systems make up the vast majority of heat engines. The basic concept of a heat engine is based on the relationships between heat, volume, and pressure of a working fluid. This fluid is generally a gas, however in some cases it may change from gas to liquid and back to gas during a cycle.
When a gas is heated, it expands; nevertheless, when a gas is contained, it expands and its pressure rises. If the bottom wall of the confinement chamber is the top of a moving piston, the pressure exerted on the piston’s surface causes it to move downward. This movement can then be employed to generate work equal to the entire force applied to the piston’s top multiplied by the piston’s distance travelled.
Refrigerators and heat pumps
Mechanical energy converters such as refrigerators and heat pumps convert mechanical energy to heat. Closed systems account for the vast majority of these. The temperature of a gas rises when it is compressed. The hot gas can then transmit heat into the environment. Because some of the heat energy was lost during the hot cycle, the temperature of the compressed gas decreases below what it was before compression when it is allowed to expand. The cold gas can then absorb heat energy from its environment.
The working principle of an air conditioner is as follows. Air conditioners do not produce cold; instead, they remove it. The working fluid is sent outside by a mechanical pump, where it is compressed and heated. The heat is subsequently transmitted to the atmosphere, usually through an air-cooled heat exchanger. The heat is then taken from the internal air via another heat exchanger before being transferred indoors to expand and cool.
Second Law of Thermodynamics
According to the second law of thermodynamics, any spontaneously occurring process will always result in an increase in the universe’s entropy (S). In simple terms, the law states that the entropy of an isolated system will never decrease over time.
The second law states unequivocally that converting heat energy to mechanical energy with 100% efficiency is impossible. When we look at a piston in an engine, for example, the gas is heated to raise its pressure and move a piston. Even when the piston moves, though, there is always some heat left in the gas that can’t be used for anything else. Heat is wasted and it needs to de dumped. In this situation, waste heat is eliminated by expelling the used fuel and air mixture to the atmosphere, or by transferring it to a heat sink in the case of an automobile engine.
Applications of Second Law of Thermodynamics
Heat always flows from a body at a higher temperature to a body at a lower temperature, according to the law. This law applies to all sorts of heat engine cycles, including Otto, Diesel, and others, as well as all types of working fluids. This law has facilitated the advancement of modern cars.
Refrigerators and heat pumps based on the Reversed Carnot Cycle are another implementation of this concept. You’ll need to offer external work if you wish to transmit heat from a lower-temperature body to a higher-temperature body. Heat produces work in the original Carnot Cycle, whereas work is provided to transport heat from a lower temperature reservoir to a higher temperature reservoir in the Reversed Carnot Cycle.
The heat from the food in the refrigerator is not automatically removed and thrown away into the warmer environment. To do this in the refrigerator, we’ll need to supply external work via the compressor.
The laws of thermodynamics apply to both air conditioners and heat pumps. By releasing the absorbed heat into the atmosphere, the air conditioner removes heat from the room and keeps it at a lower temperature. In the winter, the heat pump collects heat from the atmosphere and distributes it to the room, which is cooler.
Conclusion
The branch of science that deals with the transfer of energy from one form to another, as well as the relationship between heat and temperature, energy, and work done, is known as thermodynamics. In other words, thermodynamics is the branch of science concerned with the study of the combined effects of heat and work on changes in state of matter governed by thermodynamic principles.
Energy, like matter, is constantly conserved, which means it cannot be created or destroyed, but it can be transformed into many forms. Internal energy refers to the energy associated with a system’s molecules and includes both kinetic and potential energy. A series of energy transfers and conversions occur whenever a system changes as a result of the interaction of heat, work, and internal energy.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






