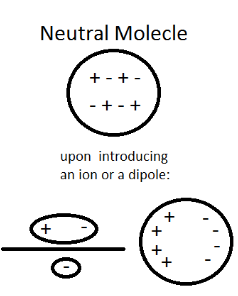
Polarisability is the ability of a molecule to attain an electric dipole moment when an external field is applied to it. The applied electric field can distort the molecule. A dipole moment is induced into a molecule when an external electric field is applied, as this field interacts with the molecule’s electron cloud and can distort it. This induced dipole moment is directly proportional to the electric field applied:
μind= αE
Where μind = induced dipole moment
E= applied electric field
α = polarisability
Hence, polarisability α = μind/E
Polarisability can be defined as the ratio of the induced dipole moment of the molecule to the applied electric field. As polarisability is directly proportional to the induced dipole moment, the greater the polarisability, the more the induced dipole moment will be and vice versa.
Polarisability of molecules
When a molecule having zero dipole moment is kept in an electric field, the centres of positive and negative charge get shifted according to the direction of the area. The molecule is said to be polarised and acquires an induced dipole moment (μind). If the molecule has a non-zero dipole moment, the induced dipole moment gets added to the permanent dipole moment already present in the molecule.
The polarisability α is a function of direction in the molecule, i.e., it depends on which direction the electric field is applied. For example, in HCl, the polarisability along the axis will be different from the polarisability perpendicular to the axis. For gases and liquids, in which molecules keep rotating, an average value of α is considered.
Polarisability plays an important role in the intermolecular interaction between molecules. The polarisability of a molecule increases with an increasing number of electrons. As the number of electrons increases, they are less tightly bound by the nuclei. An electric field can easily distort the electrons, which are less tightly bound by the nuclei. Polarisability measures how easily an electric field can distort the electron cloud, and the molecules are said to be easily polarisable.
How polarisability is induced in molecules:
When an electric field is applied to a neutral molecule, the evenly distributed charge gets separated into positive and negative charges. This induces an electric dipole moment in the molecule, making it polar. The molecule is said to be polarised.
Types of induced polarisation:
- Atomic polarisation: when nuclei are distorted to each other, it is called atomic polarisation.
- Electric polarisation: when the electron cloud is distorted, it is called electric polarisation
Examples of polarisability
Large molecules like halogen molecules are highly polarisable because they have a large electron cloud. Polarisability increases down the group. Iodine will be more polarisable as compared to chlorine. When polarisability is high, the species is said to be soft.
Anions are more polarisable than cations. Positively charged ions have their electron cloud tightly bound to the nuclei. Hence they cannot be easily distorted by an external electric field. In turn, cations can desort the electron cloud of larger molecules or ions. This is known as polarising power. The more the polarising power of the cation, the stronger is its ability to distort the electron cloud. When polarisability is low, then the species are said to be hard.
Cations with high charge density have high polarising power, and anions with high charge density have high polarisability.
Fajan’s rule and polarisability:
Fajan’s rule predicts whether a bond will be ionic or covalent.
Small cation-Large anion = Covalent
Small cation-Small anion = Ionic
Large Cation-small anion = Ionic
Large cation-Large anion = Can be ionic or covalent (depends on electronegativity)
Small cations like Be2+ and Al3+ have strong polarising power. They polarise large ions like iodine or bromine to give a covalent compound. They tend to distort the electron cloud of anions, and thus there occurs sharing of electrons rather than a complete transfer of electrons to form an ionic bond. The bond between aluminium and iodine in AlI3 will have a more covalent character than ionic. The Al3+ions can easily distort I- because of their soft nature; they will draw the electrons closer to themselves. These electrons are near the aluminium ion and neutralise the positive charge. But in the case of AlF3, the compound will be predominantly ionic. This is because F– is small and less polarisable. Al3+ ions cannot distort its electron cloud, thus leading to a more iconic character in the bond.
Similarly, consider CsF, the large cation like Cs+ does have low polarising power to polarise hard anion-like F–. Hence, CsF is also completely ionic. In the case of CsI, Cs+ cannot distort the soft I– due to its low polarising power. Hence, it also has more ionic character than covalent.
Conclusion
The ability of large atoms or ions to get distorted under external electric fields or cations is called polarisability. Large metal atoms can also be polarised, but the small anions have comparatively less polarising power than small cations. A predominant covalent character is observed when a small cation and large anion form a compound. Polarisability tends to play a major role in predicting the nature of the bond, whether it will be more ionic or covalent. Fajans rules are based on this concept of polarisability, and it categorises molecules as ionic or covalent based on their size, charge and polarisability. Hence, polarisability and electronegativity play a major role in predicting the nature of bonds in molecules.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out