Iso stands for constant and thermal stands for temperature. Isothermal processes are the processes in which the temperature is constant. Expansion takes place when the volume of the system increases or when pressure decreases. Isothermal expansion is the process of expansion in which the temperature is constant. The process maintains thermal equilibrium.
What is Isothermal Expansion?
Isothermal expansion is a thermodynamic process in which the system’s temperature does not vary.
Here, it is important to first understand thermodynamics systems and procedures.
Thermodynamics
Thermodynamics is concerned with the transfer of heat energy between the system and the environment, conversion of that heat energy into mechanical energy, and vice versa.
Thermodynamic System
A thermodynamic system is a collection of a large number of molecules or atoms enclosed within a specific region with a particular pressure (P), volume (V), and temperature (T).
‘Surroundings’ refers to everything outside this system with which heat or mass is exchanged.
Thermodynamic systems are classified into three groups based on the interconnection between a system and its surroundings:
- Open system: If both heat and mass are exchanged with the system’s environment and surroundings.
- Closed system: If there is no exchange of mass, but heat can be exchanged.
- Isolated system: If there is no heat and mass exchange with the system’s environment and surroundings.
Equation of State (for Ideal Gases)
The equation of state is the relationship between the P, V, T of the thermodynamics system. PV = nRT is the equation of state for an ideal gas with n moles.
The parameters P, V, and T are also known as thermodynamic variables.
Work Done by a Gas
Considering the volume (V), pressure (P) of the gas, and the piston’s area (A), F = PA is the force applied on the piston by gas.
Allow the piston to travel a modest distance dx during the gas expansion.
dW = Fdx = PAdx is the work done for a little displacement dx.
Since A dx = dV, the increase in gas volume is dV.
⇒ dW = P dV
or W = ∫ dW = ∫ PdV
The work done during the process is represented by the area contained within the P-V curve.
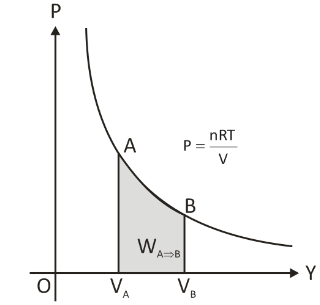
Overview of Isothermal Expansion
The temperature of the system remains unchanged during this type of expansion. There is heat exchange between both the system and the environment. The system should be compressed or expanded slowly to allow appropriate heat exchange and keep the temperature constant.
Slope of P-V curve in an isothermal process:
PV = constant = C
⇒ dP / dV = – P / V
Work done in the isothermal process:
In this process, the gas follows Boyle’s law, and work done by the gas is,
W = nRT ln Vf / Vi
[If Vf > Vi (expansion takes place), then W is positive]
[If Vf < Vi (compression takes place), then W is negative]
This is also the quantity of heat transferred when the gas volume is changed from Vi to Vf. The molar heat capacity of the system in such a process is infinite because the temperature change is zero.
Cisothermal = △Q / n△T = infinity.
Equation of Isothermal Expansion for an Ideal Gas
As discussed earlier, an ideal gas follows the equation PV = nRT. Now, for an isothermal expansion process, the system’s temperature is constant. Assuming a closed system, the number of moles, n, will also be constant.
So, the equation of isothermal expansion can be written as
PV = C
Where C is the constant.
The above equation can also be written as
T = Constant
T = 0
Assume a system under isothermal expansion. Let the initial state be represented as 1, and the final state be represented as 2.
The equation of this system can be written as
P1V1=P2V2= Constant
Isothermal Expansion Example
Sinking the apparatus in a large reservoir and performing the operation slowly could result in an isothermal process. Therefore, the system’s temperature remains the same as the reservoir’s temperature. If necessary, the reservoir and the system can exchange heat.
When gas is placed in a metal cylinder (which is an excellent conductor of heat) and the piston is moved slowly, the temperature of the gas does not vary. It maintains the same temperature as the surrounding air. The temperature rises if heat is transferred from the gas to the air through the metallic walls. The temperature drops if heat is transferred from the surrounding air to the gas through the metallic walls—the surrounding air functions as a large reservoir here.
Conclusion
Isothermal expansion is the thermodynamic process of increasing the volume or decreasing the pressure when the temperature of the system is constant. The process maintains the state of thermal equilibrium. The work done in an isothermal expansion is positive as the system does it.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






