Heisenberg’s uncertainty principle asserts that the more exactly the location is recognized, the further uncertain the momentum is, and vice versa, when the momentum is precisely known but the position is unknown. This is very different from what Newtonian Physics suggests. It asserts that given sufficient technology, all particle variables may be traced to an infinite degree of uncertainty. This is a basic principle of quantum physics that explains why a scientist cannot simultaneously measure many quantum variables. Let us learn about the importance of Heisenberg’s uncertainty principle in detail.
Origin of uncertainty principle
The dual nature of a wave particle is the major cause of the origin of the uncertainty principle. Since each particle has a wave structure, the probability of detecting particles is greater where the wave’s undulations are greatest. The wavelength becomes more ill-defined as the particle’s undulation increases, which aids in determining the particle’s momentum. This demonstrates that particles with specified positions do not have a constant or set velocity. The precise velocity is given by a particle with a well-defined wavelength. As a result, precise measurement of one quantity causes significant uncertainty in the measurement of the other.
Formula of Heisenberg’s uncertainty principle
This idea was proposed by a German scientist, Werner Heisenberg, who asserted that the position and momentum of every particle cannot be measured with infinitely high accuracy simultaneously. Similarly, the product of the errors of these two measurements has a minimal value. As a result, there is a limit for the product of energy and time uncertainties. This is because of the quantum mechanical description of nature’s intrinsic wave qualities.
This principle will not be revealed by ordinary scientific experience. This is because the uncertainties predicted by this concept are too small to be observed for ordinary items. Consequently, the product of position and velocity uncertainty is equivalent to or larger than a relatively small physical quantity, h. As a result, this product of uncertainty will only be significant for atoms and subatomic particles with very small masses.
The value of position and momentum is higher than h/4π all the time.
Formula: ∆x∆p ≥ h4
Where,
The letter h denotes the Planck constant (6.62607004 x 10-34 m2 kg / s).
∆p denotes uncertainty in the momentum
∆x denotes uncertainty in the position
Another formula to express Heisenberg’s uncertainty principle is:
∆x∆mv ≥ h4
This happens because momentum is p = mv.
When position or momentum are measured accurately, it instantly indicates a higher inaccuracy in the measurement of the other quantity.
Application of Heisenberg’s uncertainty principle
The applications of Heisenberg’s uncertainty principle are as follows:
In the nucleus, there are no free electrons
The width of the spectral lines
The quantum physics-based Heisenberg’s uncertainty principle explains several things that classical physics could not explain. One of the applications is to demonstrate that an electron cannot exist within a nucleus.
It goes like this:
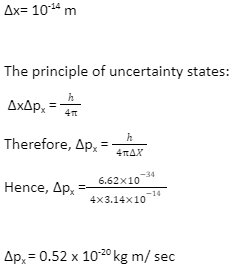
Let us suppose that there are electrons present in the nucleus. The nucleus’s diameter is about 10-14 metres. If the electron is to exist inside the nucleus, the position of the electron must be uncertain.
If the uncertainty in the electron’s momentum is the above value, then the electron’s momentum should be at least of this order, p=0.52 10-20 kg m/sec. With such a large momentum, an electron’s velocity must be similar to that of light. As a result, the following relativistic formula should be used to compute its energy:
E= √ m20 c4 + p2c2
E = √(9.1*10-31)2 (3*108)4 + (0.52*10-20)2(3*108)2
= √(6707.61*10-30) +(2.42*10-24)
E= 1.56*10-12 J
Or, E= 1.56 MeV
Consequently, if the electron happens to be in the nucleus, its energy should be in the span of 1.56 MeV. Nonetheless, electrons released from the nucleus have an energy of around 3 MeV, which differs considerably from the value obtained of 1.56 MeV. The other reason an electron cannot exist inside the nucleus is that no electron or particle in the atom has an energy greater than 4 MeV, according to experimental evidence.
Hence, it has been established that electrons do not exist within the nucleus.
Conclusion
Heisenberg’s uncertainty principle has a significant impact on how experiments are conceived and executed in science. Consider determining a particle’s momentum or position. To make a measurement, you need to interact with the particle and change its other variables. A collision between an electron and another particle, such as a photon, is required to monitor the position of an electron, for example. This will transfer some of the momenta of the second particle to the electron being measured, causing it to change. A particle with a shorter wavelength and hence more energy would be required for a more accurate determination of the electron’s position, but this would shift the momentum more during contact.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out