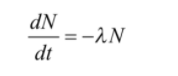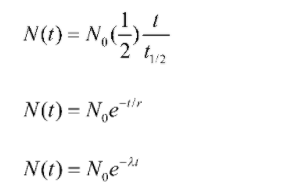Half – life or formerly known as Half – life period is one of the most common terminologies which is used in physics to define the radioactive decay of a specific sample or element over a specific period of time.
We know radioactivity is a spontaneous phenomenon. It obeys the exponential law and the lifetime of an atom can range from zero to infinity (∞). Thus, it is possible to determine the mean-life of a radioactive element. The lifespan of radioactive atoms therefore ranges from 0 to infinity. The mean-life is the sum of the lifespan of all atoms to the total number of atoms originally present.
Radioactivity
Due to the nuclear instability, the nucleus of an atom exhibits the phenomenon of radioactivity. Energy is lost through radiation emitted by the unstable nucleus of an atom. Two forces, namely the repulsive force, which is electrostatic, and the nucleus’s strong attractive forces hold the nucleus together. These forces are considered as extremely strong in nature. The probability of encountering instability increases when the size of the nucleus increases, since the mass of the nucleus becomes very large when it is concentrated. Because of this, the atoms of plutonium and uranium are extremely unstable and suffer from the phenomenon of radioactivity.
Radioactive Radiation
There are three radioactive radiations which are obtained from alpha, beta and gamma rays.
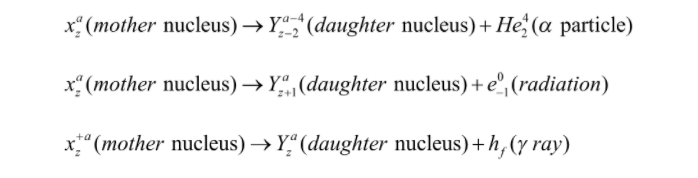
Laws of Radioactivity
Radioactivity occurs due to the decay of the nucleus.
The rate of decay of the nucleus is free from temperature and pressure.
The radioactivity depends on the law of conservation of charge.
The physical and chemical properties of the daughter nucleus differ from those of the mother nucleus.
The emission of energy from radioactivity is given by alpha particles, beta particles, and gamma particles.
The rate of decay of radioactive elements depends on the number of atoms which are present at the moment.
Radioactive Formula
The formula for radioactive is given as
The differential form of radioactive is given as
Here,
No= initial quantity of an element
λ= decay constant
t = half life time
Life Definition
Life of a radioactive element is defined as the time which is taken by the substance to get completely decomposed.
Half – Life
Half-life is usually defined as the time taken for a radioactive substance (or half of the atoms) to decompose or change into another substance. The principle was firstly given by Ernest Rutherford in 1907. It is usually denoted by the Ug or .
This concept is widely used in nuclear physics and explains how quickly atoms undergo radioactive decay. In addition, it also means how long an atom will survive radioactive decay. In addition, the half-life can help characterize any type of decay exponential or non-exponential.
Half – Life formula
The formula for half – life is given as
Here,
N(t)= quantity of substance remaining
No= initial quantity of an element
λ= decay constant
t1/2= half life
t = mean life time
Mean Life
The mean-life of radioactive elements is expected to be slightly more than half-life. If, for a given radioactive substance, half of its elements have decayed after a half-life, then a well-defined average-life can be considered that corresponds to the mean-life of the atoms.
In radioactivity, mean life is defined as the average lifetime of all nuclei of a specific unstable atomic species. Mean life is also considered as the sum of lifetimes of all the particular unstable nuclei in a given sample divided by the total number of present unstable nuclei. Mean life of a specific element of unstable nucleus is 1.443 times more than its half – life (time interval needed for the decay of half of the unstable nuclei). Lead-209 decays to bismuth-209 whose mean life is 4.69 hours and a half-life is around 3.25 hours.
Formula for Mean Life
Mean life is the sum of the lifetime of all the atoms which then divided by the total number of atoms available.
Mean life of a radioactive element is given as
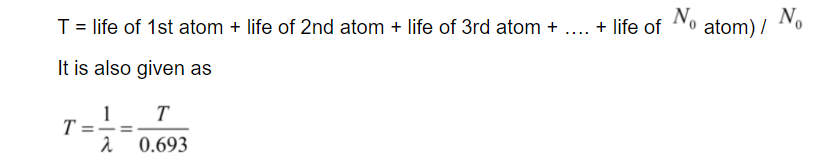
From this equation, the mean or average life is proportional to the half – life.
Conclusion
Due to the nuclear instability, the nucleus of an atom exhibits the phenomenon of radioactivity. Energy is lost through radiation emitted by the unstable nucleus of an atom.
The formula for radioactive is given as
The differential form of radioactive is given as
Half-life is usually defined as the time taken for a radioactive substance (or half of the atoms) to decompose or change into another substance.
The formula for half – life is given as
In radioactivity, mean life is defined as the average lifetime of all nuclei of a specific unstable atomic species.
Mean life of a radioactive element is given as
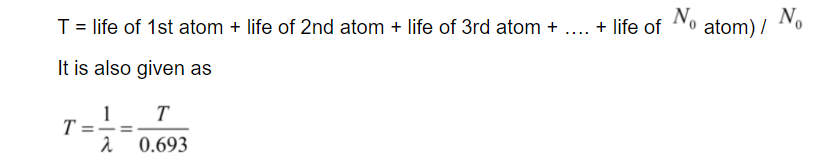
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out