The gas laws are the most fundamental laws of thermodynamics. They describe how the energy levels of atoms and molecules change with temperature, pressure, and chemical environment. They also describe how these change when a system is moving from one state to another. These laws are often used to predict the properties of a material when it is under certain conditions. Let us discuss Boyle’s Law in detail:
What is Boyle’s Law?
Statement of the Law
Boyle’s law describes the relationship between a gas’s pressure and volume at a constant temperature. The volume of a gas is inversely proportional to the pressure of a gas at a constant temperature.
The equation for Boyle’s law formula is:
V ∝ 1/P
Or
P ∝ 1/V
Or
PV = K1
V represents the gas volume, P represents the gas pressure, and K1 represents the constant. Boyle’s Law can be used to calculate the current pressure or volume of a gas and is also known as:
P1V1 = P2V2
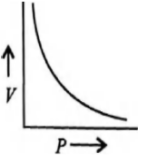
Graphical representation of Boyle’s Law
The connection between volume and pressure in a gas may be stated as Boyle’s law mathematically as follows (at constant mass and temperature).
P ∝ (1/V)
The pressure exerted by the gas is P, and the volume occupied by it is V. By adding a constant, k, to this proportionality, we can convert it into an equation.
P = k×(1/V) ⇒ PV = k
When the pressure exerted by the gas (P) is shown on the Y-axis, and the inverse of the volume occupied by the gas (1/V) is plotted on the X-axis, a straight line is formed.
Derivation and Boyle’s law formula
According to Boyle’s law, any change in the volume filled by a gas (at constant quantity and temperature) results in a difference in its pressure. Put another way, the product of a gas’s initial pressure and initial volume equals the product of the gas’s final pressure and final volume (at constant temperature and number of moles). This law can be mathematically represented as follows:
P1V1 = P2V2
Here,
P1 refers to the gas’s starting pressure.
V1 is the gas’s initial volume of occupancy.
P2 refers to the gas’s ultimate pressure.
The final volume filled by the gas is V2.
Boyle’s law formula suggests a pressure-volume connection, which may be used to get this phrase. PV = k for a certain quantity of gas at constant temperatures. Therefore,
P1V1 = k (initial pressure × initial volume)
P2V2 = k (final pressure × final volume)
∴ P1V1 = P2V2
When a gas’s container volume decreases, the equation can be used to predict the increase in pressure exerted by the gas on the container walls (and its quantity and absolute temperature remain unchanged).
Boyle’s Law examples
- Flat tires are weak and lack good form, making it difficult for a vehicle to move appropriately. The air molecules get closely packed when air is forced into flat tires with the aid of an air pump. The pressure exerted on the tire walls increases as the number of air molecules in the tire increases. Therefore, inflating flat tires is yet another application of Boyle’s rule in the real world.
- One of the most notable instances of Boyle’s law is a soda bottle filled with a combination of carbon dioxide and water. It is difficult to compress a soda can or container that has been sealed. This is because the air molecules within the container are densely packed and have little room to move. When you open a can or a bottle, some air molecules leave, allowing more air molecules to move about and compress the bottle.
- A syringe is a piece of medical equipment used to inject or remove fluids. It comprises a cylinder that holds the liquid and a plunger that controls the pressure. The volume of the fluid decreases as the plunger is pushed down, increasing the pressure. Similarly, raising the plunger increases the volume while lowering the pressure. As a result, Boyle’s law governs the operation of a syringe.
Conclusion
Experiments and observations in physics have led to the development of four fundamental principles that describe how gases behave: the law of Boyle, the law of Charles, Avogadro’s law and the law of Gay-Lussac. The experiments and observations described in this section have led to the development of gas laws. These laws are most useful in explaining the behaviour of gases under constant pressure and constant temperature. We’ve further discussed Boyle’s law and its mathematical expression that is P1V1 = P2V2.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out