Introduction
The mechanism through which nuclear reactions between light elements produce heavier atoms is known as nuclear fusion (up to iron). Significant amounts of energy are generated when the interacting nuclei are from elements with low atomic numbers (for example, hydrogen [atomic number 1] and its isotopes deuterium and tritium). The tremendous energy potential of nuclear fusion was initially harnessed in thermonuclear weapons, often known as hydrogen bombs, created in the decade following WWII.
Fusion Reaction
Fusion reactions are the primary source of energy for stars, including the Sun. As thermonuclear processes and nucleosynthesis induce compositional changes over extended time spans, the evolution of stars can be seen as a progression through numerous stages. The “burning” of hydrogen (H) commences the fusion energy source of stars, resulting in the production of helium (He). Fusion processes involving the lightest elements that burn to make helium are also used to generate practical fusion energy.
In reality, the heavy hydrogen isotopes, deuterium (D) and tritium (T), react more efficiently with each other and produce more energy per reaction than two hydrogen nuclei when they fuse. (A single proton makes up the hydrogen nucleus. One proton and one neutron make up the deuterium nucleus, while one proton and two neutrons make up the tritium nucleus.)
Nuclear Fusion
Fusion is a process in which very light nuclei fuse to generate heavier atoms.
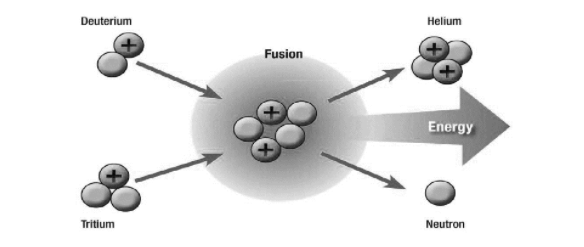
The products of fusion have a higher binding energy than the reactants. Massive amounts of energy are released as a result of the mass defect. Produces more energy per gramme of product than fission, with no by-products.
Although nuclear fusion releases huge amount of energy, it is not used for energy production as:
- The reaction currently requires more energy to initiate than it yields.
- The heat generated is so great that the containment vessels melt.
The reason why nuclear fusion requires energy is that:
- Two nuclei must be close enough for the strong nuclear force to connect them in order to combine. When positive nuclei approach, however, the electrostatic repulsion force is stronger than the nuclear force. To overcome the repulsive force, the nuclei must be extremely active.
- High temperatures (millions of degrees Celsius) are required, which is challenging to obtain while keeping the atoms contained.
Example of Nuclear Fission Reaction
Nuclear Fission
Large nuclei (mass numbers larger than 230) are prone to splitting into two or more fragments. This is referred to as fission.
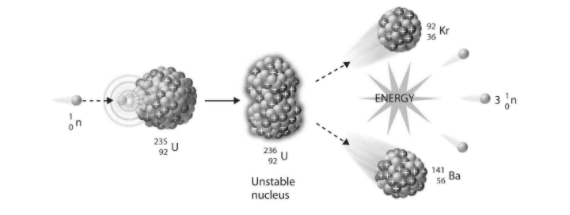
Fission is not a natural occurrence. It can only happen when a slow neutron collides with an unstable nucleus. The nucleus will divide into two almost equal nuclei during this decay process, releasing several free neutrons and a large quantity of energy.
These nuclei are isotopes of elements that are more stable. They decay radioactively if left alone, generating alpha or beta particles. Three neutrons are released on average. If they are slowed down by a moderator, they can be absorbed by other nuclei (a medium, such as graphite, heavy water, and beryllium that causes the neutrons to travel more slowly).

In this Fission reaction,
- The mass number balance is: 235+1=90+143+3
- The atomic number balance is: 92=36+56
- On average, three neutrons are released.
Difference between Nuclear fusion and nuclear fission
Nuclear Fusion | Nuclear Fission |
Nuclear fusion is a nuclear reaction in which two or more tiny atoms are fused together to generate a big atom. | Nuclear fission is a nuclear reaction in which a heavy atom is split into two or more lighter atoms. |
Nuclear fusion processes can be found all around the universe. It is used by every star to generate energy. | Fission is not a natural occurrence. |
It has a higher energy output than the fission reaction. | It generates a tremendous amount of energy. |
The procedure requires a lot of heat and pressure to take place. | Splitting an atom into two requires very little energy. |
Applications of Nuclear Fusion
In terms of nuclear fusion reactions, we are still in the experimental stage.
- Nuclear power (fission or fusion) produces no pollution because there is no burning.
- Due to the fact that fusion reactors do not produce high-level nuclear waste like fission reactors, disposal will be easier. Furthermore, unlike in fission reactors, the wastes will not include weapons-grade radioactive elements.
Nuclear fusion has the potential to solve the world’s power issue if it is used properly. When compared to fission reactions, it is clean and produces very little nuclear waste. Deuterium and Tritium, which are used as fusion fuel, are also abundant in nature. As a result, scientists believe that in the future centuries, fusion will be a viable alternative power source.
Conclusion
Nuclear fusion is a reaction in which two nuclei join to form a larger nucleus; when light nuclei fuse to generate medium-mass nuclei, energy is released.
The Q value is the amount of energy released by a fusion reaction.
Nuclear fusion discusses the reaction between deuterium and tritium that forms a fusion (or hydrogen) bomb; it also explains how the Sun generates energy, how nucleosynthesis works, and how heavy elements are formed.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






