Adsorption occurs when a liquid or gas particle adheres to the surface of an adsorbent, causing an atomic layer to develop on the adsorbate. This is distinct from absorption, in which the solute diffuses into the solid rather than on its surface. In this article, we will go through the various terms and laws related to it. The concentration of the adsorbent grows exclusively on the adsorbent’s surface during adsorption. Adsorption isotherms have proved extremely useful in studies involving environmental protection and adsorption strategies. The Freundlich and Langmuir isotherms are the two basic approaches for estimating the adsorption capacity of a particular substance.
Adsorption Isotherm
Definition
The quantity of gas adsorption per unit mass of adsorbent depends on the gas pressure. The relation between the quantity of adsorbent at a constant temperature and the pressure (or concentration for a solution) of the equilibrium gas is known as Adsorption Isotherm.
Example
Water Adsorption – In these disciplines, water adsorption at surfaces is crucial. The presence of physically or chemically adsorbed water at the surfaces of solids is referred to as water adsorption, also known as surface hydration. This is important for controlling interface characteristics, chemical reaction routes, and catalytic performance in a range of systems. In the case of physically adsorbed water, surface hydration is normally removed by the drying process, which happens at temperature and pressure conditions and results in total vaporisation of water. Chemically adsorbed water can be hydrated by dissociative adsorption or molecular adsorption.
Freundlich Adsorption Isotherms
Freundlich, a German physicist, proposed an empirical connection between the quantity of gas adsorbed by a unit mass of solid adsorbent and pressure at a given temperature in 1909. It is denoted by the following equation.
x/m = k.P1/n (n > 1)
Where ‘x’ is the mass of the gas adsorbed on the adsorbent mass ‘m’ at pressure ‘P’. ‘k’ and ‘n’ are constants that vary depending on the adsorbent and gas at a given temperature.
To demonstrate the connection, the mass of gas adsorbed per gram of adsorbent is plotted versus pressure in the shape of a curve.
Physical adsorption reduces with increasing temperature at a constant pressure. At high pressure, the curves reach saturation. Take the log of the above equation
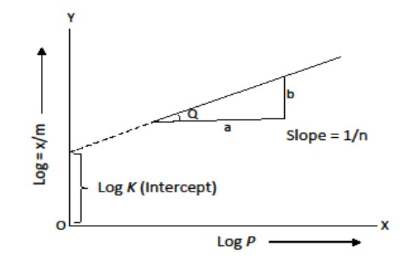
log x/m = log k + 1/n log P
Testing the Validity
We can plot log x/m on the y-axis and log P on the x-axis to test the validity of the Freundlich isotherm. The Freundlich isotherm is valid if the plot shows a straight line; otherwise, it is not. The slope of the straight line equals 1/n, but the intercept on the y-axis equals log k.
Langmuir Adsorption Isotherms
Langmuir suggested the notion of gas adsorption on a solid’s surface to be formed of elementary sites, each of which would adsorb one gas. All adsorption sites are believed to be equal, and a gas molecule’s capacity to attach to any one site is independent of whether or not the neighbouring sites are occupied. Furthermore, it is believed that adsorbed and non-adsorbed gas molecules are in dynamic equilibrium.
The following equation illustrates the Langmuir adsorption model:
Ce/qm=1/Klqmax+Ce/qmax
Ce (mg L-¹) and qm (mg g-¹) are the concentrations of the molecules at equilibrium and the amount of adsorbed molecules on the adsorbent’s surface at any time, respectively. In the equation above, The maximal adsorption capacity (mg g-1) is represented by qmax, while the Langmuir constant (L mg-1) is represented by KL.
Application of Adsorption Isotherm
- Gas masks: To absorb harmful gases, coal miners frequently employ gas masks (a device consisting of activated charcoal or a mixture of adsorbents).
- Humidity control: Adsorbents such as silica and aluminium gels are used to extract moisture and regulate humidity.
- Removes Colour from Solution: Animal charcoal eliminates colours from solutions by adsorbing coloured contaminants.
- Curing diseases: Several medicines are used in illness treatment to kill germs by becoming adsorbent on them.
- Froth floatation: A low-grade sulphide ore is concentrated by separating it from silica and other earthy materials using pine oil and a foaming agent.
- Adsorption indicators: Surfaces of some precipitates, such as silver halides, adsorb dyes such as eosin, fluorescein, and others, resulting in a unique colour at the terminus.
- Chromatographic analysis: Chromatographic analysis, which is based on the adsorption phenomenon, has many applications in analytical and industrial sectors.
Conclusion
The quantity of gas adsorption per unit mass of adsorbent depends on the gas pressure. The relation between the quantity of adsorbent at a constant temperature and the pressure (or concentration for a solution) of the equilibrium gas is known as Adsorption Isotherm. Freundlich, who was a German physicist, proposed an empirical connection between the quantity of gas adsorbed by a unit mass of solid adsorbent and pressure at a given temperature in 1909. All adsorption sites are believed to be equal, and a gas molecule’s capacity to attach to any one site is independent of whether or not the neighbouring sites are occupied.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





