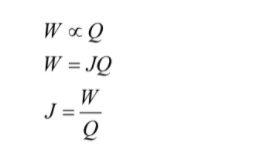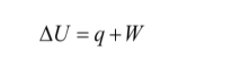By definition, heat is one of the important forms of energy for the survival of life on earth. Heat transfer from one body to another body takes place due to the temperature difference according to thermodynamics. We use heat energy for different activities, such as, Cooking, ironing, transport, recreation, etc. This form of energy also plays an important role in nature. The occurrence of wind, rain, change of seasons, etc. all depend on the gradient created by uneven heating of different regions.
Heat
As the temperature of a body increases, the vibrations of the molecules or atoms also increases. These vibrations are then transmitted from one part of the body to another part of the body. The amount of energy with which the molecules in a system vibrate is called the heat stored in that object.
According to the definition of heat, heat is the flow of energy from a warm object to a cooler object. The direction of flow of thermal (heat) energy is from the higher temperature substance to the lower temperature substance. It is due to molecules vibrating faster and transferring their energy to the slow vibrating molecules. Vibrational energy is also referred to as heat content. The heat content in the body makes the body hot or cold. The higher the heat content, the hotter the body.
A substance can absorb heat without raising the temperature by changing from one state of matter to another. During the melting process, the substance changes from solid to liquid. In the sublimation process, the solid goes into a vapor state. Boiling process, the liquid turns into vapor. Heat is a form of energy which converts into work. The amount of energy is represented in the units of work. It is expressed in joules, foot-pounds, kilowatt-hours, or calories.
Temperature
Temperature is defined as the measure of hotness or coldness of an object or a body. Celsius (C), Fahrenheit (F) scale, or Kelvins (K) are the units of temperature and Kelvin is the SI unit of temperature.
Mode of Transfer of Heat
Heat transfer can take place by three modes.
Conduction: Conduction is defined as a process where the transfer of heat takes place between atoms and molecules when they are in direct contact.
Convection: Convection is defined as a process where the transfer of heat occurs by the motion of the heated substance.
Radiation: Radiation is defined as a process where the transfer of heat takes place by electromagnetic waves.
Mechanical Equivalence of Heat
There is a simple relationship between the mechanical work done on a system and the heat generated within it. James Prescott Joule was the first to discover experimentally that the heat generated in a system is directly proportional to the mechanical work done on it. He also evaluated the constant of proportionality using a unique experiment. The constant is popularly called the mechanical equivalent of heat. This constant is also often referred to as Joule’s mechanical heat equivalent, or Joule’s constant.
Formula for Mechanical Equivalence of Heat
The mechanical equivalence of heat is given as
Here,
J = Mechanical equivalence of heat
W = work done
Q = heat produced
Therefore, from the above equation, the mechanical equivalence of heat is the work which is required to produce one unit of heat.
Mechanical energy is converted into heat, and heat is converted into mechanical energy. This important process is known as the mechanical equivalent of heat. It means that you can change the internal energy of a system by doing work on the system or providing heat to the system.
This idea of work equivalence and equivalent of heat is given in the first law of thermodynamics, according to which, the change in the internal energy of a system is the addition of the work done by the system and the heat that is added to the system.
First Law of Thermodynamics
First Law of Thermodynamics states that heat is a form of energy and therefore thermodynamic processes are subject to the law of conservation of energy. It means that the thermal (heat) energy can neither be created nor be destroyed. However, it can be transferred from one place to another and converted to one form of energy and other forms of energy.
Equation of first law of thermodynamics
The equation of first law of thermodynamics is given as
Here,
ΔU= change in internal energy
q = heat transfer
W = work done
Conclusion
Heat is the flow of energy from a warm object to a cooler object.
Temperature is defined as the measure of hotness or coldness of an object or a body.
Heat transfer can take place by three modes which are conduction, convection and radiation.
Mechanical equivalence of heat is the work which is required to produce one unit of heat.
The mechanical equivalence of heat is given as
The equation of first law of thermodynamics is given as
Heat is classified into two types: Hot (high heat content) and Cold (low heat content)
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out