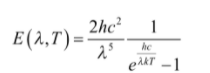A blackbody is an opaque object that emits heat radiation. A perfect blackbody absorbs all incoming light and does not reflect any of it. Such an object would seem absolutely black at normal temperature (hence the term blackbody). A blackbody, on the other hand, will begin to glow with thermal radiation when heated to a high temperature.
In reality, all objects emit thermal radiation (as long as their temperature is above Absolute Zero, or -273.15 degrees Celsius), but no item emits thermal radiation completely; rather, some wavelengths of light are better at emitting/absorbing than others. Because of these contradictions, it is difficult to analyse the interactions of light, heat, and matter using ordinary things.
Black Body Radiation
The emission of electromagnetic energy by a thermodynamically balanced object is known as black body radiation. The total energy emitted by a blackbody depends on its temperature, with a perfect blackbody absorbing and re-emitting all received radiation at any wavelength.
When an object’s temperature rises, it emits blackbody radiation, which is a common phenomena. Depending on the temperature of the object and the amount of radiation, electromagnetic radiation occupies a broad spectrum that can be both visible and invisible.
The heating element of a toaster and the filament of a light bulb is one of the most common examples. The spectral intensity of blackbody radiation increases with temperature: room temperature objects (about 300 K) emit radiation with a peak intensity in the far infrared; radiation from toaster filaments and light bulb filaments (about 700 K and 2,000 K, respectively) also peak in the infrared, though their spectra extend progressively into the visible; and the 6,000 K surface of the Sun emits blackbody radiation with a peak intensity in the visible.
Example
- The simplest example of a black body is a cavity with a hole. When light is incident on the cavity, it enters through the hole but is not reflected back by the cavity.
- A nickel-phosphorus alloy that is chemically produced and vertically aligned to the carbon nanotube arrays is a super-black material that absorbs 99.9% of light.
Emissive Powers of Black Body Radiation
A black body is one that absorbs all of the electromagnetic energy (light, etc.) that strikes it. To maintain thermal equilibrium, a black substance must release radiation at the same rate that it absorbs it, therefore it also radiates well.
We are all familiar with the radiation emitted by a heated item.
When we heat an object to roughly 1500 degrees Fahrenheit, we see a faint red glow and refer to the object as red hot. When we heat anything to around 5000 degrees Fahrenheit, close to the temperature of the sun’s surface, it radiates strongly over the visible spectrum and is said to be white hot.
By considering plates in thermal equilibrium, it is possible to demonstrate that the emissive power over the absorption coefficient must be the same as a function of wavelength, even for plates made of various materials.
If there are differences, there may be a net energy transfer from one plate to the other, which would violate the equilibrium requirement.
As a result, the black body Emissive power, E(ν,T), is a universal property that may be deduced from fundamental principles.
Rayleigh and Jeans computed the energy density (in EM waves) inside a cavity, and hence the black body emission spectrum. Their calculation was based on basic electromagnetism theory and equipartition. It not only contradicted evidence, but also claimed that all energy would be quickly radiated out as high frequency EM radiation. This was referred to as the UV disaster.
Plank discovered a formula that accurately predicted the data at both long and short wavelengths.
His formula matched the data so well that he attempted to deduce it. He was able to do this in a matter of months by assuming that energy was released in quanta with E=hν. Despite the fact that there are a huge number of cavity modes at high frequencies, the probability of emitting such high energy quanta vanishes rapidly according to the Boltzmann distribution. Plank reduced high frequency radiation in the computation, bringing it in line with the experiment. It is worth noting that Plank’s Black Body formula is the same in the limit that hν<<kT, but it goes to zero at large ν, whereas the Rayleigh formula goes to infinity. As a result, the emissive power per unit area is
Where,
k = Boltzmann’s constant, T = absolute temperature, h = Planck’s constant
Conclusion
A blackbody, on the other hand, will begin to glow with thermal radiation when heated to a high temperature. A black body is one that absorbs all of the electromagnetic energy that strikes it. To maintain thermal equilibrium, a black substance must release radiation at the same rate that it absorbs it, therefore it also radiates well. When we heat anything to around 5000 degrees Fahrenheit, close to the temperature of the sun’s surface, it radiates strongly over the visible spectrum and is said to be white hot. By considering plates in thermal equilibrium, it is possible to demonstrate that the emissive power over the absorption coefficient must be the same as a function of wavelength, even for plates made of various materials.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out