According to the first law of thermodynamics, energy can neither be created nor be destroyed; it can only be transferred from one system to another or converted to another form of energy. A chemical system can either absorb or emit heat energy.
When two bodies, which possess different temperatures, are brought near each other, then the transfer of energy in the form of heat takes place. The heat generally flows from hotter to colder bodies. The sun is the earth’s biggest source of heat energy. All the other sources derive energy from the sun. The other sources of heat energy are fossil fuels, fire, heat pumps, etc.
Introduction to the heat energy:
If sample A has a mass M and sample B has double the mass of sample A, then, to reach the same temperature, sample B would require double the heat energy compared to sample A. This is because sample B had double the mass of sample A and heat capacity depends on the mass of the substance.
The SI unit of heat is Joule per Kelvin or Joule per degree.
Use the formula below to calculate the heat capacity of a substance:
C = Q/ T
Where,
C – heat capacity
Q – heat energy supplied to bring change in temperature of the substance
T – rise in temperature
To understand the concept of heat capacity, take the example of heating iron. An iron rod heats and cools down quickly because it has very low heat capacity. However, water takes much more time to boil or cool down. This is because of the high heat capacity of water.
Hence, this example proves that heat capacity is also known as the ability of a substance to retain heat or cool down.
Heat capacity depends on the substance you are considering. It varies from substance to substance. Water has the highest heat capacity. However, you will have to consider the mass when talking about heat capacity. Large water bodies take much more time to warm up than a small amount of water in a pan, bucket or tub.
Dimensional formula of the heat energy:
The dimensional analysis uses a set of units to determine the form of an equation or, more commonly, to ensure that the result of a computation is correct as a safeguard against many common errors.
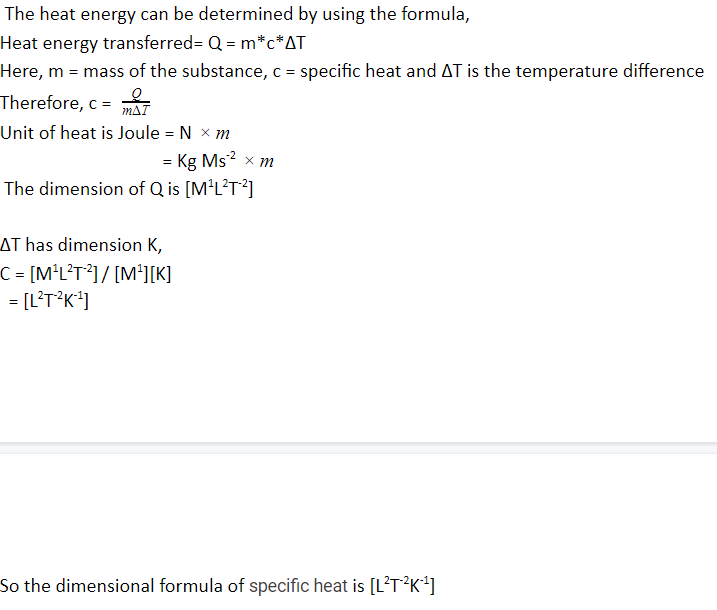
The heat energy can be determined by using the formula,
Heat energy transferred= Q = m*c*ΔT
Here, m = mass of the substance, c = specific heat and ΔT is the temperature difference
Therefore, c = QmΔT
Unit of heat is Joule = N m
= Kg Ms-2 m
The dimension of Q is [M1L2T-2]
ΔT has dimension K,
C = [M1L2T-2] / [M1][K]
= [L2T-2K-1]
So the dimensional formula of specific heat is [L2T-2K-1]
Importance of dimensional formula of heat energy
The dimensional formula of heat energy helps us to understand the physical correctness of any equation involving force.
It helps us to understand the relationship between different physical quantities involving heat energy.
It helps in converting units from one physical quantity to another.
In any relationship, the constant dimensions can be found using this analysis.
Importance of heat energy
Heat energy plays an important role in our daily life. There can be no life without heat energy. From cooking to transportation, from agriculture to industries, heat energy is intrinsically connected with our lives. Without heat energy no living beings can survive on this planet.
Conclusion
This article gives us a brief about heat energy, including introduction, dimensional analysis and its importance. Heat is a type of energy that moves between a body and its surrounding medium due to a temperature differential. Temperature is a numerical representation of the degree of hotness of a body. The thermodynamics concepts of heat and temperature work together to allow energy to flow from a hotter body to a cooler body.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out