In 1824 Nicolas Leonard Sadi Carnot developed Carnot’s theorem or Carnot rule. It deals with the efficiency of a heat engine in which there is no limit set for heat engines; they can have unlimited values. And the efficiency only depends on the hot and cold reservoir temperature of the heat engine. Of all the heat engines present, their efficiency is less than that of a Carnot heat engine. And the efficiency of a Carnot heat engine does not change with the working substance or the operation performed on it. The second law of thermodynamics is responsible for developing Carnot’s theorem. And this theory also concludes that simple heat engines or heat engines other than Carnot heat engines are less efficient.
Carnot’s theorem formula
The Carnot engine has the maximum efficiency compared to all other heat engines. The maximum efficiency is defined as the difference between the temperature of the reservoir divided by the temperature of the hot reservoir. A formula can be well established with this definition, that is:
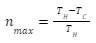
Where TH denotes the hot reservoir temperature and TC denotes the cold reservoir temperature.
The equation can also be represented as,
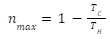
Reversible and irreversible engines
For a reversible heat engine, the efficiency is the same and equal for one which is having the same heat reservoir. And for a reversible heat engine, its process can be reversed, and it doesn’t involve any energy loss. And their efficiency will remain the same if the working between two heat reservoirs are the same.

entropy, T represents temperature, and ∫ab tells us that it is a path function.
The Carnot engine is an efficient irreversible heat engine that involves energy loss while operating.
Proof
For proving the efficiency of the Carnot engine, let us take two engines A and B operating between temperature T1and T2, where A represents an irreversible heat engine, and B represents a reversible heat engine.
If equal heat is collected by both the engines, let WA and WB represent the work output. And we know that the efficiency of irreversible heat is more than that of a reversible heat engine. That is 𝛈A > 𝛈B.
And
WA/Q > WB/Q
So,
WA > WB
The net result is that WA – WB is taken from the sink and produces an equal amount of work and it violates the second law of thermodynamics.
So the conclusion is that heat engines having irreversible processes has high efficiency is wrong. So the one working between the same temperature limit is more efficient than a reversible engine.
Meaning of Carnot’s theorem
Carnot’s theorem can be stated as a theorem proposed by Nicolas Leonard Sadi Carnot to tell us that no heat engine can be more efficient than a Carnot engine. Second law of thermodynamics is applied in Carnot’s theorem.
Carnot cycle
The Carnot cycle is created by following certain physical laws. And it is a cycle developed by taking some idealisation since it doesn’t involve any change entropy but we know that all the physical processes involve some change in entropy. So, it is an ideal cycle and it involves four consecutive operations that are first isothermal expansion, second isothermal compression expansion, third adiabatic expansion, and fourth adiabatic compression, and then it will move to the initial stage.
In this way, a cycle containing four operations is present in the Carnot cycle. In Isothermal expansion, the temperature is kept constant. And adiabatic expansion is a process in which no heat enters or leaves the system in which the process is performed. Isothermal compression is the decrease in volume without any temperature change on it. While adiabatic compression is the expansion in which pressure decreases in that process.
Application of Carnot’s theorem
Carnot’s theorem finds application in many fields. Some of the common and beneficial applications of the Carnot engine are:
- The heat engines in which the thermal energy is used to convert work apply the Carnot theorem.
- Refrigerators or refrigeration works on this to produce a cooling effect.
- The turbines or steam turbines used in ships work based on the Carnot theorem.
- A combustion vehicle’s engine that is a combustion engine works on this theorem.
- The turbines present in engines of aircraft follow Carnot’s theorem.
Conclusion
Carnot’s theorem proposed by Nicolas Leonard Sadi Carnot gives us an idea about how efficient a Carnot engine is compared to other heat engines, and also it will give an expression for finding the efficiency of heat working between two hot reservoirs. It has many applications in the physical world. Many engines are worked out with the evolution of this theorem. And it also works based on thermodynamics. A Carnot cycle has been developed in this way in which four successive operations are performed in a cyclic rotation. It is an idealised concept that is very different from the real world.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






