At temperatures above absolute zero, all normal matter generates electromagnetic radiation, which is referred to as thermal radiation because it represents a conversion of a body’s internal thermal energy into electromagnetic energy. Normal matter, on the other hand, absorbs electromagnetic radiation to some extent. A blackbody is an object that absorbs ALL radiation falling on it, at all wavelengths. When a blackbody is kept at a constant temperature, its emission has a frequency distribution that is proportional to the temperature. Blackbody radiation is the name for this type of emission.
A blackbody at ambient temperature appears black because the majority of the energy it emits is infrared, which is invisible to the naked eye. As the human eye is unable to distinguish light waves at lower frequencies, a black body viewed in the dark at the lowest just barely observable temperature appears gray, despite the fact that its objective physical spectrum peaks in the infrared range. When it gets a little hotter, it turns a dull red colour. As the temperature rises, it turns yellow, white, and eventually blue-white.
Blackbody radiation has a distinct, continuous frequency spectrum that is dependent exclusively on the body’s temperature in experiments. Actually, we can be a lot more specific:
1.A body emits radiation at a specific temperature and frequency in the same way that it receives it.
2 Gustav Kirchhoff confirmed this statement: the main point is that we assume a specific body can absorb better than it emits, it will absorb radiation from other bodies better than it radiates energy back to them in a room full of objects all at the same temperature. This means it will heat up as the rest of the room cools down, which is in direct violation of the second rule of thermodynamics. To avoid violating the second rule of thermodynamics, a substance must produce radiation just as well as it absorbs the same radiation at a particular temperature and frequency.
Any body that is above absolute zero will radiate to some extent, with the intensity and frequency distribution of the radiation varying depending on the body’s detailed structure. To begin analysing heat radiation, we must first define the body that is radiating: the simplest feasible situation is an idealised entity that is a perfect absorber, and hence also a perfect emitter (based on the above logic). So, how can we build the perfect absorber in the lab? Kirchhoff had a brilliant concept in 1859: a little hole in the side of a huge box is a great absorber, since any radiation that passes through it bounces around inside, absorbing a lot of energy with each bounce, and has little chance of ever escaping. So, we can do it backwards: make an oven with a tiny hole on the side, and the radiation that comes out of the hole should be as close to a perfect emitter as we can get
The Law of Stefan-Boltzmann
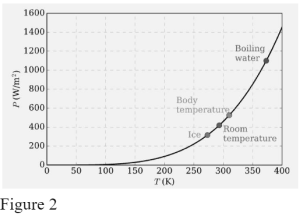
The Stefan-Boltzmann Law (1879), which states that the total power (i.e., integrated over all emitting frequencies in Figure 1.1.3) radiated from one square metre of black surface goes as the fourth power of the absolute temperature (Figure 2), was the first quantitative conjecture based on experimental observations.
P=σT4 where,
P is the total quantity of radiation released per square metre (Watts/m2) by an item.
The Stefan-Boltzman constant (5.6710-8 Watts m-2K-4) is a constant in physics.
T is the object’s absolute temperature (in K)
Boltzmann discovered the T4behaviour in 1884 by applying classical thermodynamic reasoning to a box filled with electromagnetic radiation and utilising Maxwell’s equations to relate pressure to energy density. That is, the little quantity of energy escaping through the hole (Figure 2) would have the same temperature dependency as the intensity of the radiation inside.
Wien’s Law of Displacement
Wien’s Displacement Law was the second phenomenological finding from the experiment. The dominant (peak) wavelength, or colour, of light emitted by a body at a given temperature is determined by Wien’s law. The frequency at which the emitted radiation is most strong varies with the oven temperature . The frequency is, in fact, directly related to the absolute temperature:
vmax~T
5.8791010Hz/K is the proportionality constant
Following Boltzmann’s thermodynamic logic, Wien deduced this law theoretically in 1893. Langley, an American astronomer, had previously spotted it, at least semi-quantitatively. Everyone is familiar with this upward change in max with T: when an iron is cooked in a fire , the initial visible radiation (at roughly 900 K) is deep red, the lowest frequency visible light. At very high temperatures (10,000 K or more), where the peak in radiation intensity has shifted beyond the visible into the ultraviolet, the hue changes to orange, yellow, and finally blue as T increases.
Conclusion
As a result, the amount of energy radiated by a dark body should grow as the temperature rises. We conclude that the total energy of radiation emitted by a black body is proportional to a monotonously increasing function of thermodynamic temperature only on the basis of these reasons
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






