Alfred Werner was the son of a factory man J.A. Werner. He was born on 12th December in 1866 at Mulhausen in Alsace, France. Alfred Werner has always had a keen interest in the field of Chemistry ever since he was a kid. When he was only 18 years of age, he performed his first independent Chemistry research.
Alfred Werner also served in the military for one year in Karlsruhe. He attended his lectures at the Technical High School in the same city he was born and brought up in.
Alfred Werner’s name will be forever linked to the theory of coordination that he devised, as well as his work on the spatial interactions of atoms in molecules, the roots of which were laid in the work he did for his doctorate thesis in 1892 when he was just 24 years old.
Alfred Werner received the Nobel Prize in the field of Chemistry in 1913 for his coordination theory of transition metal ammine complexes.
Oxidation State (Oxidation Number)
The oxidation state or number of a compound is defined as a number assigned to each element in a chemical combination that represents the number of electrons lost or gained by an atom of that element in the given chemical compound.Coordination Number
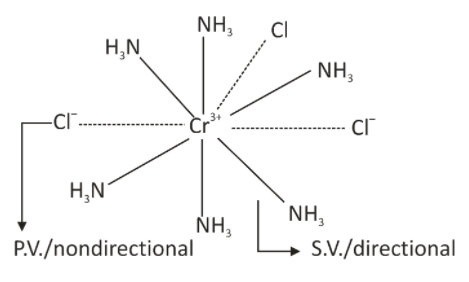
The coordination number is defined as the number of atoms/ions surrounding a central atom in a crystal molecule or complex compound. The primary Valency satisfies the Oxidation Number. The secondary Valency satisfies the Coordination Number.Werner’s theory of coordination Compounds
Alfred Werner’s theory of coordination Compounds tells us that-- Metals inherit two types of valencies, namely- primary Valency and secondary Valency or ionizable Valency and non-ionizable Valency.
- Each atom of an element tends to satisfy the valencies of both the primary Valency as well as the secondary Valency.
- A definite geometrical arrangement is given to the compound, in which the ligands that satisfy the non-ionizable Valency in space are directed towards a fixed position.
Postulates of Werner’s theory of coordination Compounds
The basic postulates of Werner’s theory of coordination Compounds, which Sir Alfred Werner, the Nobel Prize Winner in Chemistry, stated are listed below-- The ions or groups bound with the help of secondary linkages to the metal have a characteristic of spatial arrangements that correspond to different coordination numbers.
- The secondary valencies are also called non-ionizable valencies. These valencies are then satisfied using neutral molecules or ions, which are negative in charge. The secondary Valency equals the coordination number and does not change for a metal.
- The primary valencies are normally ionizable and are satisfied by negatively charged ions.
- In coordination, compound metals show two types of valencies (links): namely, primary Valency and secondary Valency or ionizable Valency and non-ionizable Valency.
- In a complex compound, the central metal atom exhibits two types of Valency: Primary Valency, also called Principal Valency → Corresponds to the Oxidation State → Electrons lost or gained, and Secondary Valency, also called Auxiliary Valency → Corresponds to the Coordination Number → Ligands around the Central Metal.
Drawbacks of Werner’s Theory of Coordination Number
- It doesn’t explain why simply a few components form coordination compounds.
- It fails to tell us and explain why coordination molecule bonds have directional characteristics.
- It fails to explain and tell us the colour of coordination compounds magnetic and optical properties.
Characteristics of Secondary Valency
- In coordination and compounds, the secondary Valency of a metallic atom in a compound is equivalent to the coordination number of that metal.
- A secondary Valency of metal is either satisfied by the anions or by the neutral molecules only or with the help of anions and neutral molecules both.
- For Example-
- The elements that satisfy the non-ionizable are called ligands.
- When writing the structure of a complex compound, the items satisfying the secondary Valency and the metal are written inside the coordination brackets.
Characteristics of Primary Valency
- The Primary Valency of the metallic atom in a complex compound is the same as the oxidation state or oxidation number of that metal. For Example- the primary Valency of Cobalt-atom in all the four Co(III) Amines is equal to +3.
- The primary Valency of metal in a complex compound is always satisfied by anions. For Example- The primary Valency of Cobalt-atom in each of the four ammines is equal to +3 and is satisfied with the help of three Cl- ions. [Co(III)(Ligands)]3+ → [CoIII(Ligands)]Cl3.
- The anions that satisfy the primary Valency are written outside the coordination brackets, while the anions which satisfy both the valencies are written inside the coordination brackets. [Metals (Ligands)]Anions.
- The items satisfying the primary Valency can be present inside or outside the coordination brackets.
- Primary valency is represented by dotted line( ………… )
- Secondary valency is represented by solid line( _____ )
- Ligand which satisfy both primary valency as well as secondary valency are represented by solid with dotted line ___________ ………..
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





