Let us consider an example of chemical equilibrium:
2NO2 (g)→ N2O4 (g)
NO2 is reddish-brown coloured, while N2O4 is colourless. If NO2 is kept in a sealed, evacuated glass vessel at 25℃, it starts getting converted to colourless N2O4. However, after a while, it can be seen that the intensity of the brown colour gets constant, signifying that the concentration of NO2 is no longer changing. When such a stage is reached, we can say that a state of equilibrium has been reached.
Chemical equilibrium in a reversible reaction can be defined as the state at which two opposing reactions occur at the same speed. The reaction doesn’t stop.
If the reaction is of type: jA + kB ⇌ lC +mD
The law of mass action can be represented by the equilibrium expression given below:
K = [C]l [D]m∕ [A]j [B]k
Here, K is the equilibrium constant.
Types and examples of chemical equilibrium
There are two types of chemical equilibria: (i) Homogeneous and (ii) Heterogeneous.
Homogeneous equilibrium
The reversible reactions in which all the reactants and products are present in the same state, i.e., only one phase is present, are homogeneous reactions. When equilibrium is achieved in such chemical reactions, it is known as homogeneous equilibrium.
Homogeneous reactions can be further classified into three types:
First type: Number of molecules are not changed in a reaction. Examples include:
(a) H2 (g) + I2 (g) ⇌ 2HI (g)
(b) CH3COOH(l) + CH3CH2OH (l) ⇌ CH3COOCH2CH3 (l) + H2O (l)
Second type: There is an increase in the number of molecules in a reaction. Examples include:
(a) PCl5 (g)⇌ PCl3 (g) + Cl2 (g)
(b) 2NH3 (g) ⇌ N2(g) + 3H2(g)
Third type: There is a decrease in the number of molecules in a reaction. Examples include:
(a) N2(g) + 3H2(g) ⇌ 2NH3 (g)
(b) 2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
The value of the equilibrium constant is also dependent on certain factors in these reactions.
- The mode of representation of the reaction: Concentrations of products are kept at the numerator, whereas the concentrations of reactants are kept at the denominator.
A + B ⇌ C+ D
Here, equilibrium constant K can be written as
K= [C][D]/[A][B]
However, if we consider the reverse reaction,
C + D⇌ A+ B
Now, K’ = [A][B]/[C][D] = 1/ K
- Stoichiometric representation: When a reversible reaction can be written in the form of 2 or more stoichiometric equations, the value of K will differ in each case. In general, if we multiply a balanced equation with any value n, the new equilibrium constant will be now equal to Kn.
A+ B⇌ C+ D, K= [C][D]/[A][B]
nA +nB ⇌ nC + nD, K’ = [C]n[D]n/ [A]n[B]n = Kn
- Use of partial pressures in place of concentrations: When the reaction takes place in the gaseous phase, partial pressure can be used instead of concentration as the partial pressure of a substance is proportional to its concentration in the gas phase.
From the ideal gas equation, PV= nRT
Or P= (n/V) RT = CRT
Where C is the number of moles of gas per unit volume
For the general reaction
jA + kB⇌ lC + mD
Let the partial pressures be PA , PB, PC and PD, respectively at equilibrium.
So,, Kp = (Pcl)(PDl) / ( PAj)( PBk)
= ( CC× RT)l ( CD RT)m / (CA RT)j ( CBRT)k
= [C]l[D]m/[A]j[B]k ×(RT)(l+m)-(j+k) = K (RT)∆n
Where ∆n = difference in the sums of coefficients for gaseous reactants and products.
Units of Equilibrium Constant
K has no unit for a reaction where the reactants and products are equimolar.
In general, unit of K = [M]∆n
Where M = mol litre-1 and ∆n= number of moles of gaseous products- number of moles of gaseous reactants.
Similarly, the unit of Kp = [atm]∆n
Heterogeneous equilibrium
The reversible reactions where the components occur in more than one phase are known as heterogeneous reactions. The equilibrium achieved in heterogeneous reactions is called heterogeneous equilibrium.
Examples include:
(a) CaCO3 (s) ⇌ CaO (s) + CO2 (g)
(b) H2O (g) + C (s) ⇌ H2 (g) + CO (g)
If we consider the example of decomposition of calcium carbonate and try to derive its equilibrium expression, we get
K’ = [CO2][ CaO]/ [CaCO3]
However, it has been determined experimentally that the concentrations of pure solid and pure liquid phases don’t appear in the expression of the equilibrium constant for a heterogeneous reaction. Here CaCO3 is a pure solid, and its activity is taken as unity. Similarly, the activity of CaO is also unity as the reference states are pure CaCO3 and pure CaO, respectively.
So, the equilibrium expression can be written as
K= [CO2](1)/ (1) = [CO2]
And Kp = PCO2 (1) / (1) = PCO2
To generalise, we can say that in the case of a pure solid or a pure liquid, activity is always 1.
Conclusion
In summary, we can say that there are two types of chemical reactions, i.e., reversible and irreversible reactions. Chemical equilibrium is a characteristic property of a reversible reaction. The equilibrium state is the state where the rate of the forward reaction and backward or reverse reaction are equal.
However, the reaction doesn’t actually stop, so it is dynamic in nature.
There are two types of chemical equilibrium- heterogeneous and homogeneous.
The equilibrium taking place in a reaction where every component is in the phase is called homogeneous equilibrium, whereas, if two or more phases are present, the equilibrium is heterogeneous.

where k is a constant whose value is determined by the volume of the gas V and its temperature P.
According to Boyle’s law, the product of the volume and pressure of a given amount of gas at a certain temperature is constant based on the preceding equation.
Let T be the temperature and P2 be the pressure of a particular quantity of gas. Now, if the gas’s pressure at temperature T is increased to V2, resulting in a volume V2, according to Boyle’s law, the gas’s volume will be V2.
P1 V1 = P2V2
They are constants, when mass and temperature are constant.
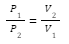
P1= Initial pressure exerted by the gas
P2= Final pressure exerted by the gas
V1= Initial volume occupied by the gas
V2= Final volume occupied by the gas
Examples of Boyle’s law
Respiration: Our lungs use Boyle’s law during respiration. When you breathe in, your lungs expand because they are filled with air. As the volume increases, the pressure level decreases. Similarly, when air is deflated, the lungs contract, reducing volume and increasing pressure. The change in pressure and volume is both instantaneous and periodic.
Soda bottle: One of the best examples of Boyle’s law is a soda bottle filled with a mixture of carbon dioxide and water. It is difficult to squeeze a sealed beverage can or container. This is because the air molecules inside the container are tightly packed and have little room to move. When you open a can or bottle, some air molecules leave the container, allowing room for more air molecules to move in and squeeze the bottle. The change in pressure as a function of the volume can be clearly seen in this example.
Scuba diving: When diving underwater, it is important to ensure that the volume and pressure ratios are balanced to avoid illness or injury. You feel great pressure when entering or approaching the depth of the body of water. The solubility of gases in the human blood increases with high pressure. As it rises or moves up, the pressure in the blood begins to drop, and the gases in the blood begin to expand. As a result, the diver must ascend slowly to avoid damage. Boyle’s law is based on the relationship between pressure and volume.
Graphical representation of Boyle’s law
A direct line going via the starting place for a plot of P vs 1/V at a steady temperature for a fixed quantity of fuel line might be a graphical illustration of Boyle’s Law.
A rectangular hyperbola is a plot of P vs V at constant temperature for a specific mass of gas.
A straight line parallel to the PV axis is used to plot P (or V) versus PV at constant temperature for a specific mass of a gas.
Conclusion
Boyle’s law regulation is very important as it explains how gases behave. It proves past a shadow of a doubt that fuel line strain and quantity are inversely proportional. When you press down on a fuel line, the quantity shrinks, and the strain rises.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





