In 1939, Ryutaro Tsuchida in Japan proposed the idea of the relation of molecular geometry and the number of valence electron pairs (paired or unpaired). In 1940, Nevil Sidgwick presented it in a Bakerian Lecture. This concept was refined in more detailed theory by Ronald Gillespie and Ronald Sydney Nyholm. From the electron pair, the shape of the molecule that surrounds the molecule, to predict this VSEPR theory is used. The theory assumes that molecules take shape in the valence shell and that an atom’s electronic repulsion is minimized. In VSEPR theory, the single bonding group combines a double bond or triple bond. So, let us understand more about VSEPR theory postulates, VSEPR theory, and the main points of VSEPR theory.
VSEPR Theory
The Valence Shell Electron Pair Repulsion Theory is also known as the VSEPR theory. It is predicted that there is always a repulsion between the pairs of valence electrons in all atoms. To minimize the repulsion in the atom, the electron pairs are arranged in such a manner. This arrangement of atoms determines the geometry of the resulting molecule. It increases its stability and decreases the molecule’s energy, and the molecular energy is determined.
It is also known as the Gillespie-Nyholm theory, after its two main founders, Ronald Nyholm and Ronald Gillespie.
VSEPR theory Postulates
The postulates of VSEPR theory are as follows
- The atoms belonging to one molecule are linked to the central atom of polyatomic molecules (molecules that are made up of three or more atoms)
- The shape of a molecule is decided by the total number of valence shell electron pairs
- The valence shell electron pair repulsion theory can be applied to each resonance structure of a molecule
- The electron pair must be localized in the surface so that the distance between them is maximized so that the valence shell can be thought of as a spare
- In two bond pairs, the strength of repulsion is weakest, but it is strongest in the two lone pairs
- In the central atom, the electron pairs are closer to each other
- They will start repelling each other, which results in an energy increment of the molecules
- When the electron pairs are lying far from each other, the repulsion between them is less, resulting in energy decrement of molecules
- To have a distorted shape of the molecule, the central atom should be surrounded by the bond pair of electrons and the lone pair of electrons
- The asymmetrical shape of a molecule can be expected when the bond pair of electrons should surround the central atom
- To minimize the electron-electron repulsion and maximize the distance between them, the electron pair tends to orient themselves in that way
VSEPR Theory’s Limitations
Valence shell electron pair repulsion theory has some limitations, which are
- Isoelectronic species( which have the same number of electrons) the VSEPR theory fails to explain this. Having the same number of electrons, but spices vary in shape
- The actual structure of halides group 2 elements is bent one
- But the Valence shell electron pair repulsion theory predicts the shape of halides group 2 elements is a linear structure
- Another limitation of the Valence shell electron pair repulsion theory is that it does not have any explanation for the compound of transition metals
- The structure was not described properly by this theory
The Predicted Shape of Molecules
To decide the shape of the molecule these steps must be followed
- The least electronegative atom must be considered as the central atom(to share the electrons with the other atom belonging to the other molecule, this atom has the highest ability)
- The total number of electrons must be counted which belongs to the outermost shell of the central atom
- Using the bonds with the central atoms and the total number of electrons belonging to other atoms must be counted
Find the three molecules attached to a central atom and arrangements
- To obtain the valence shell electron pair number or VSEPR number these two values must be added
What is the Valence Shell Electron Pair Repulsion Number?
The shape of the molecule is described by the VSEPR number
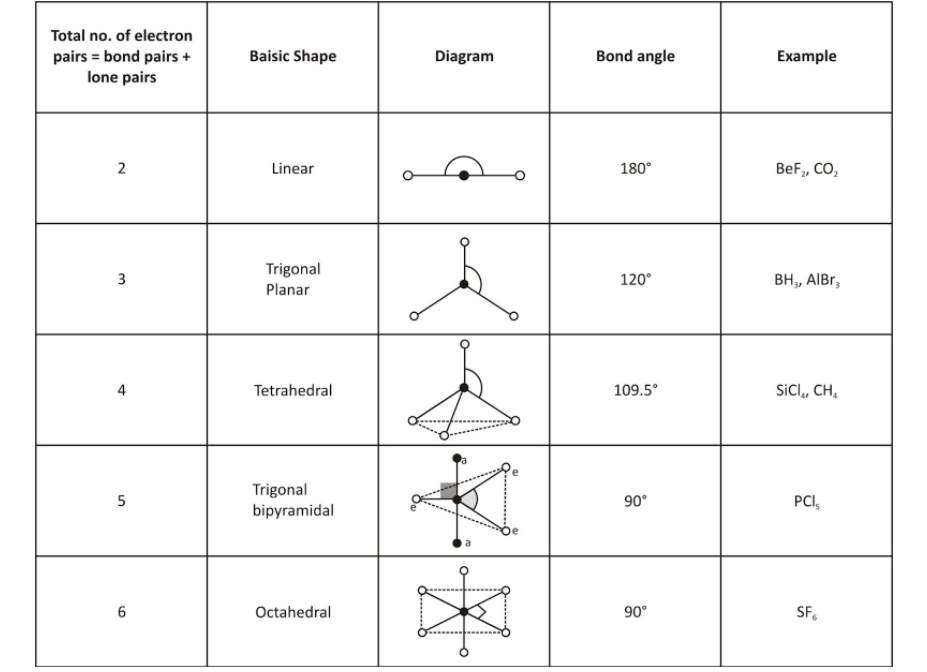
To obtain the exact bond angle between the atoms in a molecule the VSEPR theory can be used.
Molecule’s Linear Shape
If we have a compound that has no lone pairs of electrons, like BeH2, it will form into a linear compound as the hydrogens will repel each other as much as possible to make a straight line.
Like BeF2 do have lone pairs of electrons on the fluorines, 6 electrons each to be exact. This compound is still linear because the equal number of lone pairs will repel each other so much that the farthest away they can be from each other is when the molecule rests at 180 degrees.
VSEPR theory helps to predict the geometry of most polyatomic molecules and ions (positive and negative) by focusing only on the number of electron pairs around the central atom, ignoring all other valence electrons present.
Molecule’s trigonal planar shape; Arranged them so that the repulsion between the electrons can be minimized.
Molecule’s Tetrahedral Shape
The atoms lie in the same plane, in two dimensional-molecules. Then, we will get the square planar geometry.
We will get a tetrahedral molecule if we consider this condition for a three-dimensional molecule.
Molecule’s Trigonal Bipyramidal Shape
Along the equator of the molecule, three positions lie in trigonal bipyramidal. The axis perpendicular to the equatorial plane two positions lies in trigonal bi[pyramidal.
Conclusion
The VSEPR theory is used to predict the electron pairs’ arrangement around the central atom of molecules. Anatom, which is bonded to two or more other atoms, is known as the central atom, and the atom bonded to only one other atom is known as the terminal atom. IN VSEPR theory, the electrons pair repel each other whether they are in a bond pair or a lone pair or not. To minimize the repulsion, electron pairs spread themselves as far as from each other. Valence shell electron pair repulsion can predict the shape of all compounds having the central atom, as long as the central atom is not metal.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




