A chemical reaction’s equilibrium constant explains the relationship between the products and reactants when a chemical reaction reaches equilibrium.
In a balanced chemical equation, the equilibrium constant is defined as the ratio of the product of the molar concentrations of the products to the product of the molar concentrations of the reactants. Each concentration term must be raised to an exponential power equal to the stoichiometric coefficient
What is the kp equilibrium constant in a chemical reaction?
If both the reactants and the products are in the gaseous state, the equilibrium constant is expressed in partial pressure of the reactants and products.
The equilibrium constant kp is determined by atmospheric pressure and is expressed by the expression:
qQ+ rR ⇌ sS+ tT
The formula for equilibrium constant would be:

Units of partial pressure are:
- Atm
- Bar
- Pascal
What is kc?
Kc represents the equilibrium constant in a reversible reaction.
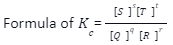
Where:
[S] is the molar concentration of the product
[T] – T is the molar concentration of the product
[Q] – Q is the molar concentration of the reactant
[R]– R is the molar concentration of the reactant
Relationship between Kp and Kc
The equilibrium constants of gaseous mixtures are Kp and Kc. The equilibrium constants do not include the concentrations of single components such as liquids and solids, and they do not have any units.
Consider the following reversible reaction:
qQ + rR ⇌ sS + tT
The equilibrium constant of the reaction, expressed in concentration (mol/litre), can be expressed as:
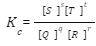
If the equilibrium involves gaseous species, the concentrations may be expressed in partial pressures of the gaseous substance. The equilibrium constant in terms of partial pressures may be given as:
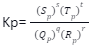
Where pQ, pR, pS and pT represent the partial pressures of the substance Q R, S and T respectively. If gases are assumed to be ideal, then according to the ideal gas equation:
PV = nRT
p = nRT / V
Where p is pressure in Pa
n is the amount of gas in mol
V is Volume in cubic metre
T is temperature in Kelvin
n/V = concentration, C
or
p = CRT or [gas] RT
If C is in mol dm-3 and p is in bar, then R = 0.0831 bar dm3 mol-1 K-1.
Let us suppose a general reaction:
qQ + rR↔ sS + tT
The equilibrium constant will be given as:
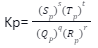
Now, p = CRT
Hence,
pQ= [Q] RT
Where [Q] is the molar concentration of the reactant Q
Similarly,
PR= [R] RT
PS= [S] RT
PT = [T] RT
Where [R], [S] and [T] are the molar concentration of R, S and T, respectively.
Substituting these values in expression for Kp, i.e., in equation (1).
Kp = [([S] RT) s ([T] RT) t]/[([Q] RT) q ([R] RT) r]
= [S] s[T] t (RT) s+t/[Q] q [R] r (RT) q+r
= [S] s [T] t (RT) s+t – q+r/[Q] q [R] r
= Kc (RT) s+t-q+r
= Kc (RT) ∆n
Kp and kc will change accordingly with the number of moles of gas molecules. This can be shown by giving various cases.
Case 1:
If Δng = 0, when the change in the number of moles gas molecules in the equation is zero.
Then Kp = Kc
Case 2:
When the number of moles of gas molecules increases, i.e. if delta ng > 0, then Kp > Kc
Case 3:
When the number of moles of gas molecules decreases, i.e. if Δng < 0, then
Kp < Kc
Equilibrium constant units for kp and kc
For the general reaction: qQ+ rR ⇌ sS + tT
Kc =(MolL-1)(s+t) \(MolL-1)(q+r) = (MolL-1)(s+t)-(q+r) = [Mole L-1]△n
Therefore the unit for Kc= [Mole L-1]△n
Kp= (atm)(c+d) \(atm)(a+b) = (atm or bar)(c+d)-(a+b) or (atm or bar)n
Therefore, the unit for Kp= atm or bar.
Questions on the unit of the equilibrium constant
Example 1:
For the reaction:
N2O4(g) ⇌ 2 NO2(g)
The concentration of the equilibrium mixture at 293 K of N2O4 is 5 x 10-8mol/L, and of NO2 is 2 x 10-6mol/L. Find the value of the equilibrium constant.
Applying the formula: k = [NO]2\[N2O4]
Taking the concentrations with regard to standard state concentration of 1 mol/L:
K= 8×10-5
Example 2:
For the reaction:
N2 (g) + 3H2 (g) ⇌ 2 NH3(g)
If pN2 = 0.30 atm, pH2 = 0.20 atm, and pNH3= 0.40 atm, then what is the value of Kp?
Using the formula: Kp = p[NH3]2\p[N2].p[H2]3
Kp= 13.3 atm
Conclusion
The equilibrium constant has various applications, which includes prediction of the extent and direction of the reactions. It also helps in the calculation of the equilibrium concentration.
The relationship between Kpand Kc is Kc(RT) ∆n. The relation works under three major cases, when the number of moles of reactant and products are either equal, positive or negative. The unit of equilibrium constant determines if the reaction will move forward or backwards depending on the number of moles or partial pressure of the given products and reactants.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





