Salts are built of positive and negative ions, bound together by the force of attraction of their opposite charges. Suppose energy needed to break their ionic bonds is lower than the energy given off by an interaction of the ions with a solvent (i.e. water H2O). In that case, the salts thus dissociate and even interact with solvent and then get dissolved. At first, the salt dissociates quickly, and the reaction generally goes one way:
NaCl(s) → Na+(aq) + Cl-(aq).
The more salt gets dissociated, the more saturated the solution becomes.
In the end, there are so many ions that are dissolved in the water that the reverse reaction begins:
Na+(aq) + Cl- (aq) →NaCl(s)
The reverse reaction reduces the rate at which the water absorbs the subsequent salt particles. Finally, the speed of the reverse reaction reaches the concentration dissociation rate of the ions, which does not change with time. The solution reaches equilibrium under the given conditions and is called a saturated solution.
Salt solubility is the amount of salt added to water for a solution to reach equilibrium.
Sparingly soluble salts
A poorly soluble salt has very low solubility. The solubility of a substance is defined as the amount of a substance that can be dissolved in 100ml of water.
The product of the molar concentration of its ions in a saturated solution is the definition for the solubility product of an electrolyte at a particular temperature. Add salts with the same solubility product.
Examples of sparingly soluble salt
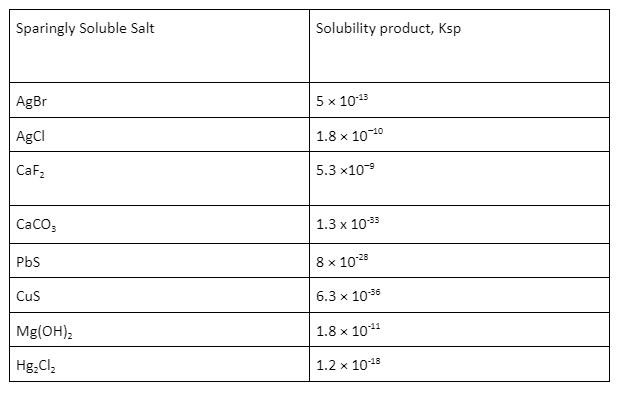
Solubility product constant is the simplified equilibrium constant (Ksp) defined for equilibrium between solids and its separate ions in a result. Its value indicates the degree to which an emulsion dissociates in water. The advanced the solubility product constant, the further answerable the emulsion.
The Ksp expression for a swab is the product of the attention of the ions, with each attention raised to a power equal to the measure of that ion in the balanced equation for the solubility equilibrium.
what does that mean?
Solubility product constants are used to describe impregnated results of ionic composites of fairly low solubility. A logged result is in a state of dynamic equilibrium between the dissolved, separated, ionic emulsion and the undissolved solid. As for every result, at equilibrium in given conditions, we can write an expression like the one below for Tableware chloride
solubility equilibrium general expression

solubility of a slightly soluble salt
It is very difficult to measure the solubility of a sparingly soluble salt. However, knowing its solubility product can help determine its solubility at any temperature.
The relationship between solubility (nmol/L) and solubility product (Ksp) depends upon the nature of the salt.
What is the Relation between Molar Solubility of a Sparingly Soluble Salt and Solubility Product?
For the saturated solution of a sparingly soluble salt named AB, The given following solubility equilibrium may also exist.
AB(s, std.soln.) A(aq)++ B-(aq)
If the molar solubility of the salt is s, then [A+ ] = s mol dm-3 ,
[B-] = s mol dm-3
Hence ,
Ksp = [A+ ][B-]
= (s mol dm-3) (s mol dm-3)
= s2 mol2 dm-6
Example. The solubility of silver chloride given in water at a temperature of 25°C is found to be 0.00179 g per litre. Calculate its solubility product at 25°C.
Solution :
Solubility of silver chloride
= 0.00179 g dm
= 0.00179 g dm-3 /143.5 g mol
= 0.0000125 mol dm-3
The given dissolved salt would also be present here in the given form of ions so that,
[Ag+] = [CI-] = 0.0000125 mol dm-3
Ksp= [Ag+][Cl-] = (0.0000125 mol dm -3 ) 2
= 1.56x 10-10 mol2 dm-6
Application of Solubility product principle
- Solubilities of sparingly soluble salts determination
- Predicting precipitation reactions
- Fractional precipitation
- Insoluble salt preferential precipitation
- Precipitation of soluble salts
a. Cleansing of soluble salts
b. Release of soap - Inorganic analysis
a. Precipitation of sulphides
b. Precipitation of hydroxides - Other precipitation reactions
- Dissolving precipitates of phosphates, carbonates, sulphides Etc.
Conclusion:
The solubility of a sparingly soluble salt can be calculated using the solubility product principle, which is the ionic product of the salt at saturation. The sparing solubility salt contains some ions in the solution and some residue of the undissolved salt. A solubility product is the same as an ionic product, wherein the undissolved salt and the dissolved ions are in dynamic equilibrium.
The value of the solubility product of the sparingly soluble salts is used in various applications, including finding its solubility, which is otherwise difficult to analyse, in qualitative analysis and predicting precipitation reactions and even in the process of precipitation of soluble salts.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





