What is the Rosenmund reduction Mechanism?
The Rosenmund reduction mechanism is a term used to explain how acyl chlorides get selectively reduced into aldehydes. Karl Wilhelm Rosenmund first reported the reaction in 1918. Karl Wilhelm Rosenmund was a famous German chemist credited with the Rosenmund-von Braun reaction.
The Rosenmund reaction is a hydrogenation procedure where molecular hydrogen reacts with an acyl chloride in the presence of a catalyst, which is usually palladium on barium sulphate. Palladium on barium sulphate is therefore also referred to as the Rosenmund catalyst.
Interestingly, the Rosenmund catalyst, palladium on barium sulphate, is also used for the decarboxylation of aliphatic esters to produce biodiesel. Of course, it is also used in hydrogenation reactions.
What is the Role of Catalyst Poison in the Rosenmund Reaction?
As mentioned above, the Rosenmund reaction is when acid chlorides get converted into aldehyde through catalytic reduction with palladium and barium sulphate in either quinoline or sulphur. The addition of barium sulphate helps reduce the activity of the palladium because of barium sulphate’s low surface area.
This prevents the reaction from over-reduction. Suppose there is a need for furher reduction in the activity of palladium. As is the case with more reactive acyl chlorides, poison is also added to completely deactivate the palladium catalyst.
Some common catalyst poisons used to restrict palladium’s activity in the Rosenmund reaction are thiourea and thioquinanthrene. This need for further deactivation of palladium arises because the aldehyde formed from the reduction of the acyl chloride also gets reduced to primary alcohol by the reaction.
This is an unwanted reaction in the Rosenmund technique because the primary alcohol reacts with the remaining acyl chloride to form an ester. The Rosenmund catalyst, which is palladium on barium sulphate, is prepared by reducing palladium (II) chloride solution with the inclusion of reducing agent formaldehyde in the presence of barium sulphate.
Deactivation is necessary for the reaction because the system needs to reduce the acyl chloride but not the produced aldehyde. So if further reduction continues to occur, it will create primary alcohol. This primary alcohol would react with the remaining acyl chloride and produce an ester.
This is why catalyst poison is required to achieve the desired product, which is an aldehyde. If the poisoned palladium is not used, the reaction will produce an ester instead of aldehyde.
How does the Rosenmund Reaction Work?
Let us look at the mechanism of the Rosenmund reaction:
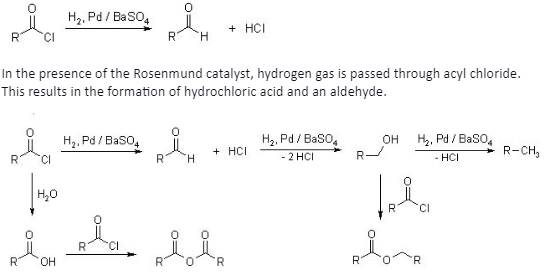
The aldehyde that is formed needs to undergo another reaction with the catalyst, palladium over barium sulphate. This causes the formation of alcohol, which can further be generated into an alkane.
Now, the Rosenmund catalyst is positioned, which stops any further reduction after the desired product has been achieved. There are a variety of positions that are used for this process. However, the most commonly used poisons are thioquinanthrene and thiourea.
What are the Applications of the Rosenmund Reduction Reaction?
You may be wondering what the applications of reducing the reaction are? Here are some of the applications of the Rosenmund reduction reaction:
Rosenmund reaction is primarily used for the production of aldehydes. It is also used for producing aryl aldehydes or alkyl. Furthermore, it is used for creating saturated fatty aldehydes.
Are there any Limitations of the Rosenmund Reaction?
It is possible to prepare many aldehydes by using the Rosenmund reduction reactions. However, it is not possible to prepare formaldehyde. This is because formyl chloride remains unstable at room temperature and cannot be used.
Conclusion
The Rosenmund reaction helps convert acid chlorides into aldehydes using catalytic reduction with palladium on barium sulphate. A catalyst poison is added in the Rosenmund reaction in order to completely deactivate the palladium catalyst. It is possible to prepare many aldehydes using the Rosenmund reaction.
To know more about the Rosenmund reaction and the role catalyst poison plays in response to preparing for competitive exams, candidates should go through a wide range of study material provided by Unacademy for gaining a better understanding. So, begin your preparation for entrance examinations from today itself with Unacademy.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





