The capability of carbon to establish bonds with other atoms by participating its valence electrons is known as tetravalency. This principally means that carbon has four valence electrons ( external electrons that are available for forming bonds with other titles), carbon is called tetravalent. A tetravalent element is an essential with four electrons available to form bonds with other atoms.
![]() As carbon possesses atomic number 6, it means that the carbon atom has a total of 6 electrons. In simple ways, its electronic configuration can be written as 2,4. It means it has 4 electrons in the outermost shell. Carbon obeys the octet rule and forms 4 covalent bonds with other atoms to get a stable electronic configuration. Thus, carbon is tetravalent (It means the valency of carbon is 4.) and can form 4 covalent bonds with not only other atoms but other carbon atoms as well. This is called tetravalency of carbon. It is a unique property of carbon as it forms very strong covalent bonds which makes carbon compounds exceptionally stable in nature. The ability of carbon to form covalent bonds with other carbon atoms is called catenation. Due to this property carbon can form long straight, branched, and cyclic chains. Carbon can form single, double, and triple covalent bonds with other carbon atoms.
As carbon possesses atomic number 6, it means that the carbon atom has a total of 6 electrons. In simple ways, its electronic configuration can be written as 2,4. It means it has 4 electrons in the outermost shell. Carbon obeys the octet rule and forms 4 covalent bonds with other atoms to get a stable electronic configuration. Thus, carbon is tetravalent (It means the valency of carbon is 4.) and can form 4 covalent bonds with not only other atoms but other carbon atoms as well. This is called tetravalency of carbon. It is a unique property of carbon as it forms very strong covalent bonds which makes carbon compounds exceptionally stable in nature. The ability of carbon to form covalent bonds with other carbon atoms is called catenation. Due to this property carbon can form long straight, branched, and cyclic chains. Carbon can form single, double, and triple covalent bonds with other carbon atoms.
Tetravalency of Carbon
As carbon possesses atomic number 6, it means that carbon grain has a total of 6 electrons. . Carbon obeys the sextet rule and forms 4 covalent bonds with other particles to get a stable electronic configuration. Thus, carbon is tetravalent (It means valency of carbon is 4.) and can form 4 covalent bonds with not only other particles but other carbon particles as well. This is called tetravalency of carbon. It’s a unique property of carbon as it forms truly strong covalent bonds which makes carbon mixes exceptionally stable in nature. The capability of carbon to form covalent bonds with other carbon particles is called concatenation. Due to this property carbon can form long straight, banged and cyclic chains. Carbon can form single, double and triple covalent bonds with other carbon particles. Still, 2S2, 2P2 .Electronic Configuration and Excited State in Carbon
If you see the electronic configuration of carbon grain in detail also you will find that the electronic configuration of carbon at ground state is 1s2 2s2 2p2.Above electronic configuration of carbon shows that carbon has only 2 unmatched electrons. We know that carbon has 4 covalent bonds. Also the generality of the excited state comes. When carbon grain gets agitated it shows following electronic configuration 1s2, 2s1, 2p3Size of a carbon snippet
Although there’s no specific description, the composites in solid, liquid or gassy state which contain covalently clicked carbon titles in their motes are known as organic composites.Hybridization of Carbon
Hybridized orbitals are helpful in describing the shape of molecular orbitals piecemeal from being a major part of valence bond proposition. For illustration, in methane whose chemical formula is CH4, a set of sp3 orbitals develops by combining one s-orbital and three p-orbitals on the carbon snippet. These orbitals direct towards the four hydrogen titles placed at the vertices of a regular tetrahedron.Tetravalence of Carbon
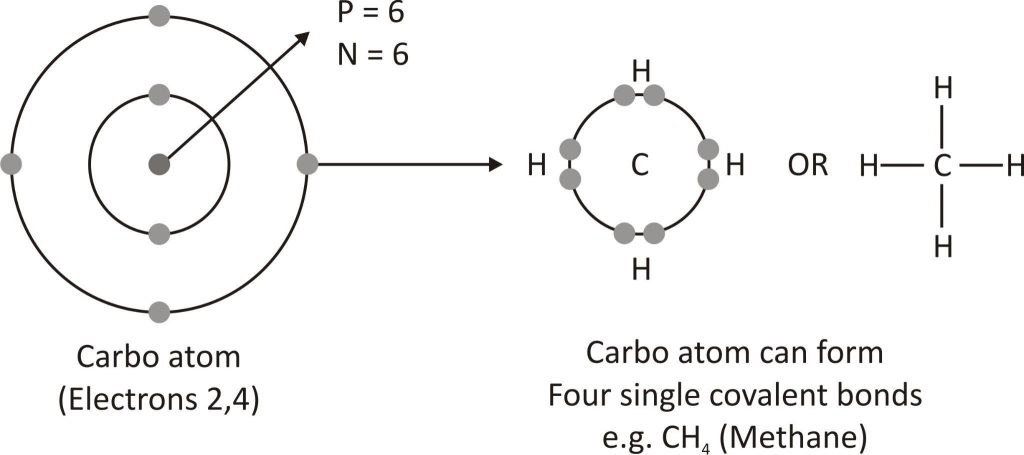 As carbon possesses atomic number 6, it means that the carbon atom has a total of 6 electrons. In simple ways, its electronic configuration can be written as 2,4. It means it has 4 electrons in the outermost shell. Carbon obeys the octet rule and forms 4 covalent bonds with other atoms to get a stable electronic configuration. Thus, carbon is tetravalent (It means the valency of carbon is 4.) and can form 4 covalent bonds with not only other atoms but other carbon atoms as well. This is called tetravalency of carbon. It is a unique property of carbon as it forms very strong covalent bonds which makes carbon compounds exceptionally stable in nature. The ability of carbon to form covalent bonds with other carbon atoms is called catenation. Due to this property carbon can form long straight, branched, and cyclic chains. Carbon can form single, double, and triple covalent bonds with other carbon atoms.
As carbon possesses atomic number 6, it means that the carbon atom has a total of 6 electrons. In simple ways, its electronic configuration can be written as 2,4. It means it has 4 electrons in the outermost shell. Carbon obeys the octet rule and forms 4 covalent bonds with other atoms to get a stable electronic configuration. Thus, carbon is tetravalent (It means the valency of carbon is 4.) and can form 4 covalent bonds with not only other atoms but other carbon atoms as well. This is called tetravalency of carbon. It is a unique property of carbon as it forms very strong covalent bonds which makes carbon compounds exceptionally stable in nature. The ability of carbon to form covalent bonds with other carbon atoms is called catenation. Due to this property carbon can form long straight, branched, and cyclic chains. Carbon can form single, double, and triple covalent bonds with other carbon atoms.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





