Borax is also called sodium borate, sodium tetraborate, and disodium tetraborate. It is a useful boron compound, a salt, and a mineral obtained from boric acid. It is a component used in many cosmetics, detergents, and enamel glazes. It is also used in making buffer solutions in biochemistry. Sometimes, it can be used as an insecticide when mixed with boric acid.
History of borax
In the 1900s, borax was heavily used by the Philippines in mining gold. It was first discovered in dry lake beds. It was used to import silk roads to the Arabian peninsula in the early 8th century. Later in the 19th century, borax came into regular use when a borax company began to market the applications of borax.
Structure of borax
The word borax is not only used for the compound but is also used for other related products or minerals that usually differ only in their crystal structure. Some of them are mentioned below-
- Na2B4O7·5H2O, sodium tetraborate pentahydrate
- Na2B4O7, anhydrous sodium tetraborate
- Na2B4O7·10H2O, sodium tetraborate decahydrate
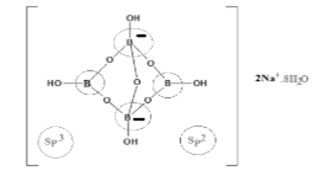
Talking about the chemical structure, the borax contains [B4O5(OH)4]2− ions. The structure contains two three-coordinate boron centers with two four-coordinate boron centers.

Borax is mainly found in evaporite deposits. It is obtained by the process of repeated evaporation. The mist commercial deposits are found in turkey and it has also been found in some parts of southwestern united states and the Atacama desert in Chile. It can also be made synthetically from other compounds of boron. The natural borax is refined by a process of recrystallization which is very common.
Properties of borax
Reactions
Borax is converted into boric acid and other products which have many real-life applications.
When reacting borax with hydrochloric acid, it yields boric acid which is shown by the following reaction.
Na2B4O7·10H2O + 2 HCl → 4 H3BO3 + 2 NaCl + 5H2O
When borax is put into a flame, it converts into a green-yellow color. Due to this reason, it is not being used in fireworks as it produces a yellow color of sodium. On the other hand, it is used to color methanol flame into a transparent green color.
Borax is highly soluble in ethylene glycol and moderately soluble in diethylene glycol and methanol. It also gets soluble in acetone. Talking about cold water, borax is very poorly soluble in it but its solubility can be increased with increasing temperature.
Reaction with sodium hydroxide –
Na2B4O7 + 7H2O + 2NaOH 🡪 4Na[B(OH)4]
Physical Properties of Borax
- It is easily soluble in water
- Molar mass is 381.38 (decahydrate) and 202.22 (anhydrous)
- Its anhydrous boiling point is 1,575℃
- It is white
- Density-2.4g/cm3 (anhydrous) and 1.73 g/cm3 (decahydrate)
- The melting point is 75 ℃ (decahydrate) and 743 ℃ (anhydrous)
Uses of borax

Borate-based detergent
Household products
Borax is used in many household products such as in laundry, hand soap and tooth bleachings. Borax powder is a detergent that can be harmful when swallowed.
pH buffer
Borate ions are used to make buffer solutions in laboratories.
Co-complexing agent
Borax is a good source of borate which is being used for its co-complexing ability with other agents in water to form various substances. ]
Water-softening agent
Borax is for water softening even if it does not have a high affinity for the hardness cations. The chemical reaction for water softening is as follows.
Ca2+ (aq) + Na2B4O7 (aq) → Ca B4O7 (s)↓ + 2 Na+ (aq)
Mg2+ (aq) + Na2B4O7 (aq) → Mg B4O7 (s)↓ + 2 Na+ (aq)
Here, the sodium ions are not responsible for hard water. This process is only suitable for both permanent and temporary types of hardness.
Flux
In the process of welding, Borax when mixed with ammonium chloride is used. It is used as a flux. It helps lower the melting point of iron oxide. It is also mixed with water to be used as a flux in soldering jewelry metals. Borax is also considered good for pre-tinning tungsten.
Small-scale gold mining
Borax was extensively used for extracting gold in small scale minings. It has replaced highly toxic mercury. This method was less expensive and quite environmentally friendly.
Flubber
Materials such as slime and flubber were made by cross-linking borax with polyvinyl alcohol. It is also known as liquid borax.
Food additive
Borax is used in some food additives. In some countries, it is banned but still used in many countries.
Other uses of borax
- A useful ingredient in enamel glazes.
- Components of ceramics, pottery, and glass.
- It is a moth proofing 10%solution used in wool
- Fire retardant
- Used as an antifungal compound for cellulose insulation
- Used for prevention of stubborn pests.
- Used as a precursor for boric acid.
- Important ingredients in starch, dextrin, and casein
- Plotide detoxification
- Used as a flux to melt heavy metals and alloys
- Act as a curing agent for snake skins.
- Used in forge welding by blacksmiths.
- Used to make indelible ink used in dip pens.
Toxicity
As per a study, borax is not acutely toxic. Botan was previously used in insecticides in the United States even after various restrictions. Late in 1986, these restrictions were removed as it was declared non-toxic.
According to the EPA, borax compounds contain low toxicity that’s why they should be spared from the requirement of sufferance.
Conclusion
This was everything that one needed to learn about Borax. There are several other topics that one needs to prepare for exams. For all those topics, you can check out various articles on Unacademy. It has covered all topics for every exam. Unacademy has valuable resources that can help you score high marks in exams.
Borax is one of the basic and most important concepts while preparing for class 12th and even for national level entrance examinations such as NEET, JEE Mains, or Advance. Considering the past papers, several questions have been asked on this topic. It holds a significant place in the syllabus and can help you achieve high marks.
Unacademy can help you with quick notes that can help a lot in exams. Kickstart your preparation journey today with Unacademy!
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





