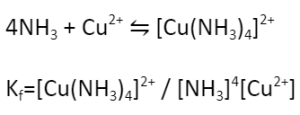Every chemical compound wants to attain maximum stability, which holds for coordination compounds. It refers to the extent of association between the metal ion and the ligands when they are at equilibrium. The stability can be expressed by the formation constant, which is the ratio of the concentration of product and reactants raised to the power of their stoichiometric constant.

The complex is formed by Lewis acid-base interaction. The stronger the interaction, the more stable the complex would be.
Stability of Complexes Importance
The concept of complexes’ stability helps us determine their reactivity. Stability is inversely proportional to reactivity. The more stable a compound is, the less reactive it would be. As stated above, the stability of complexes can be determined by finding the formation constant, denoted by Kf.
Let’s understand that using an example:
If the value of Kf is high, that means the concentration of product in the solution would be high.
A metal ion does not exist in the solution freely. It exists in the solvated form, i.e., it is surrounded by solvent molecules. So, the ligand molecules compete with the solvent molecules for coordination with the metal centre.
![]()
Moreover, all the ligands do not coordinate with the metal at once. The coordination happens in a stepwise manner, wherein the first ligand coordinates first, followed by the second ligand, followed by the third ligand, and so on. The formation constant can be determined for each step :-
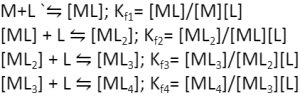
Here Kf1, Kf2, Kf3, and Kf4 are stepwise formation constants. To write the overall formation constant for the overall reaction M+4L `⇋ [ML4], we can multiply the stepwise formation constants.
So, Kf = Kf1 x Kf2 x Kf3 x Kf4
In most cases, the magnitude of the formation constant decreases as the number of ligands coordinated to the metal centre increases.
Factors affecting the stability of complexes
depends on the following factors:
- Charge on the metal ion: It is directly proportional to the stability of complexes. Generally, the greater the charge on the metal ion, the smaller is the size of the ion. This implies that the charge/radius ratio of the metal ion is large, which imparts more stability to the coordination compound. This is why the stability of complexes having Fe3+ as the metal ion is more stable than that containing Fe2+.
- Size of the metal ion: It is inversely proportional to the stability of complexes. If we talk about the 3d series, then as the size of the metal ion increases (considering the metal ion to be bivalent), the stability of complexes is found to decrease.
![]()
As we move across the period, the ionic radius decreases (due to an increase in the number of protons in the nucleus, which increases the greater force on the valence shell). The stability, hence, increases across the period.
- Chelate Effect: Multidentate ligands exhibit this effect as they form a ring-like structure around the metal ion. They have multiple donor sites which can donate electron density to the metal simultaneously, resulting in the formation of an enclosed ring. Chelates increase the stability of complexes.
- Macrocyclic Effect: When ligands that already have a ring form complexes with metal, they tend to be more stable. This is called the macrocyclic effect. The ligand heme in our body exhibits a macrocyclic effect.
Conclusion
In this article, we discussed the stability of complexes, how to determine them, why it is important, and what factors it depends on. The stability of complexes depends on both the nature of the metal ion and that of the ligands. It is crucial as we can tune the stability of complexes by modifying either the metal or the ligand. Moreover, the formation of complexes is not a one-step process, but it happens through a series of reactions. Experimentally, stability is determined by Job’s method, which involves UV-visible studies of the compound. Let’s do a few stability questions now.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out