The Periodic Table’s s-block elements are those in which the last electron reaches the outermost s-orbital. Because the s-orbital can only hold two electrons, the s-block on the Periodic Table is separated into two groups (1 & 2).
Periodic Group 1 of the Periodic Table comprises the elements lithium, sodium, potassium, rubidium, caesium, and francium. Together, they are known as alkali metals. They are called so because they create extremely alkaline hydroxides when they react with water. Group 2 elements include beryllium, magnesium, calcium, strontium, barium, and radium.
Element | Symbol | Electronic Configuration |
|---|---|---|
Beryllium | Be | [He] 2s² |
Calcium | Ca | [Ar] 4s² |
Strontium | Sr | [Kr] 5s2 |
Barium | Ba | [Xe] 6s2 |
Radium | Ra | [Rn] 7s2 |
Magnesium | Mg | [Ne] 3s2 |
Electronic Configuration
Outside the noble gas core, all alkali metals contain one valence electron ns1.
These elements have the most electropositive metals due to the loosely held s-electrons in the outermost valence shell. They quickly lose electrons, resulting in monovalent M+ ions. As a result, they are never encountered in nature in a free condition.
Chemical Properties
Due to their huge size and low ionisation enthalpy, alkali metals are extremely reactive. The reactivity of these metals rises as they progress through the group.
Air Reactivity
Alkali metals tarnish in dry air, owing to the development of oxides, which then react with moisture to generate hydroxides. They produce a lot of oxygen as they burn and, as such, produce a lot of oxides.
Lithium produces monoxide, sodium produces peroxide, while the other metals produce superoxides. Only in the presence of massive cations such as K, Rb, and Cs is the superoxide O2 – ion stable.
Water Reactivity
Alkali metals react with water to generate hydroxide and dihydrogen.
Although lithium has the highest negative E value, its interaction with water is less intense than that of sodium, which has the lowest negative E value among the alkali metals.
Lithium’s behaviour is explained by its tiny size and extremely high hydration energy.
Other metals in the group have violent reactions with water.
Proton donors, such as alcohol, gaseous ammonia, and alkynes, also react with them.
It has the largest hydration enthalpy, which explains its significant negative E value and reducing power.
Reactivity with Dihydrogen
At around 673K (lithium at 1073K), alkali metals react with dihydrogen to generate hydrides. All hydrides of alkali metals are ionic solids which have extremely high melting points.
Halogen Reactivity
Alkali has a high reactivity to halogens. Metals react violently with one other. M+ halogens combine to generate ionic halides. Lithium halides, on the other hand, are fairly toxic covalent. This is due to the high Lithium-ion polarisation capability. The Li+ ion battery ion is relatively tiny in size and has a high proclivity to disrupt the electron cloud around the ion of a negative halide. The most covalent element is lithium iodide.
Nature’s Reduction
Alkali metals are potent reducing agents, with lithium the most powerful and sodium the least. The entire change is represented by the standard electrode potential (E), which quantifies the decreasing power:
Because of the tiny size of its ion, lithium possesses the largest hydration enthalpy, which explains its significant negative E value and reducing power.
Alkali Metal Solutions in Liquid Ammonia
Alkali metals can be dissolved in liquid ammonia, producing deep blue solutions that are naturally conductive.
The blue colour of the solution is caused by the ammoniated electron, which absorbs energy in the visible area of light and, so, gives the solution a blue colour. Because these solutions are paramagnetic, they progressively release hydrogen, leading to the creation of amides.
The blue colour turns to bronze in concentrated solutions and becomes diamagnetic.
Applications
Lithium metal is used to manufacture useful alloys, such as ‘white metal’ bearings for motor engines, aircraft components, and armour plates. It is a component in thermonuclear reactions. Lithium is also employed in the production of electrochemical cells. Sodium is utilised to create a Na/Pb alloy, which is required for the production of PbEt4 and PbMe4.
These organolead compounds were once employed as anti-knock additives in gasoline. However, modern automobiles utilise lead-free gasoline. In fast breeder nuclear reactors, liquid sodium metal is employed as a coolant. Potassium is essential in biological processes. Fertilisers include potassium chloride. In the production of soft soap, potassium hydroxide is employed. It is also a great carbon dioxide absorber. Caesium is used in the creation of photoelectric cells.
Conclusion
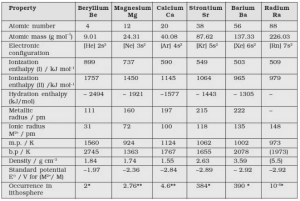
Beryllium ion is the most soluble, and as its size increases, so does its solubility. Therefore Barium ion is the least water-soluble alkaline earth metal ion. The ionic composition and size of a substance influences its solubility in water.
Smaller ions have a higher charge density and can be dissolved by a greater number of water molecules. This results in a greater enthalpy of hydration and more stable hydrated ions.
Example:
Solubility of Be2+ > Solubility of Mg2+ > Solubility of Ca2+ > Solubility of Sr2+ > Solubility of Ba2+
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





