Transfer of energy is one of the most observed phenomena in the world. Since energy cannot be created nor be destroyed, its transfer governs many of the foundational laws of our universe. Heat is a form of energy that we all have observed in our daily lives, and it is also the first form of energy that humans learnt to use.
The conduction of heat between two materials, or two slabs in series, holds the key to understanding how heat energy moves from one point to another. The conduction of heat helps us to understand how a rod gets heated up on heating its one end, and it also helps us to understand how the sun manages to keep Earth a habitable planet even from such a long distance. Conduction is one of the most basic ways heat can move from one point to another. But how does this conduction happen? Let us delve deeper.
Conduction of Heat
Conduction of heat revolves around the concept of transfer of heat from one point to another through direct collisions of subatomic particles. The movement of electrons in atoms and the collisions of microscopic particles is the primary way heat is transferred through conduction.
As a form of energy, heat at one point on the body increases the internal energy at that point. The increase in internal energy causes the atoms to get kinetically excited, increasing the vibrations inside the body. The vibrations at one point of the body in the molecules cause the neighbouring molecules to get excited too.
In this manner, the vibrations from one set of molecules are transferred to its neighbouring set and so on until it reaches the other end of the body. This increases the overall internal energy of the body, and therefore the temperature of the body also increases.
Steady State
The conduction of heat causes the temperature of the body that is subjected to heat to rise too. Conduction is much like a reaction, and a point can be achieved such that the amount of heat entering a body is equal to the amount of heat leaving the body.
The conduction that happens in such a state is called steady-state conduction. It is a special form of conduction in which the temperature distribution by space in a body is completely constant. Conduction of heat directly results from the temperature difference between two ends of a body. In the steady-state of conduction, the temperature difference that is the driving force behind this conduction, does not exist anymore. This results in the temperature field of the body or the spatial distribution of temperature of the body being constant.
What this means is that if we were to pick at random a section of the body, and take the temperature of the cross-section at a point that is normal to the flow of heat, then that temperature will be constant. Partial derivatives give us an idea of the changes that occur in a body. The partial derivative of heat with respect to space may yield a nonzero or a zero value, but the partial derivative of heat with respect to time, which indicates a change in temperature, will be zero.
A steady-state of conduction is analogous to the flow of electric current in a body. The heat that enters a body can be assumed as heat current, and all conventional laws of direct current conduction can be applied. The temperature difference is the driving force of conduction and is analogous to voltage. The body’s thermal conductivity is similar to the resistance of a wire, and the incoming heat is equal to the current that flows through a conductor.
Slabs in Parallel
Let us understand the conduction of heat between two slabs that are placed in parallel with each other. Let us have two slabs, A and B, that are placed in parallel with each other. Let T1 be the temperature at the one side of slab A, and T2 be the temperature at the end of slab A.
Similarly, T1 is the temperature at the start of slab B, and T2 is the temperature at the end of slab B. Let k1 be the thermal conductivity of slab A, and k2 be the thermal conductivity of slab B. Let L1 and L2 be the lengths of slab A and slab B, respectively. Let R1 and R2 be the thermal resistivity of slab A and slab B.
Let Q be the heat that is entering the system. Now let Q1 and Q2 be the heat that is passing through slab A and slab B, respectively. Since both the slabs are in parallel to each other, we can say that:
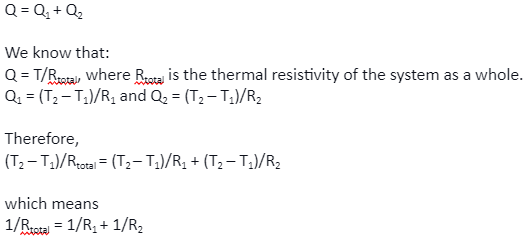
Therefore, when two slabs are kept in parallel, the total thermal resistivity is the sum of the reciprocal of the thermal resistivity of each of the slabs. The result is analogous to resistors that are connected in parallel.
Conclusion
The movement of electrons in atoms and the collisions of microscopic particles is the primary manner in which heat is transferred from the conduction of heat.
The steady state of conduction is a special form of conduction in which the temperature distribution by space in a body is completely constant. Conduction of heat directly results from the temperature difference between two ends of a body. In the steady state of conduction, the temperature difference that is the driving force behind this conduction does not exist anymore. This results in the temperature field of the body or the spatial distribution of temperature of the body being constant.
For two slabs in parallel at steady-state conduction, the reciprocal of the thermal resistivity of the two slabs add up analogously to the connection of two resistors in parallel in an electrical circuit.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



