Resonance effect is the polarity formed in a molecule as a result of contact between a lone pair of electrons and a pi bond, or the interaction of two pi bonds between two nearby atoms. In molecules with conjugated double bonds or at least one lone pair of electrons and one double bond, the effect is observable. The bigger the number of resonance contributors, the stronger the resonance stabilisation effects, and the more stable the species is, according to the resonance effect. So, in order to anticipate whether the resonance effect is present or not, we usually have to build “new” resonance structures (contributors) based on the existing ones.
Example of Resonance Effect
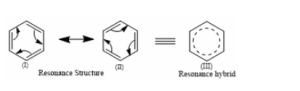
In the case of Benzene:
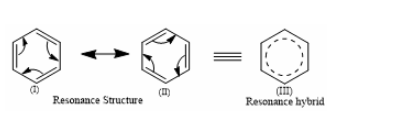
Three C-C single bonds with a bond length of 1.54 A and three C=C double bonds with a bond length of 1.34A are found in the aforementioned structures (I) and (II). However, it was discovered that all six carbon and carbon bonds are identical, and a 1.39 A intermediate C-C and C+C bond was discovered. The poor reactivity of halogen in vinyl bromide can be explained further by the phenomena of resonance.
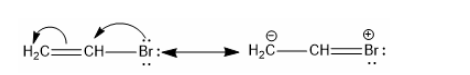
Resonance energy is the difference between the real molecule and the more stable canonical form.
Application of resonance effect
The high utility of resonance theory and its worth comes from the fact that it maintains the simple and unsophisticated form of structural representation.
Stability of carbocation
The carbocation that conjugates a positive charge with a double bond tends to be more stable. The allylic carbocation is more stable than the comparable alkyl cation because of the resonance structure. The resonance structures are formed when the negative electrons of the conjugated double bonds are delocalised, which increases their stability. The stability will be great if the resonating structure is great.
Carbanion of stability
The availability of double bonds or an aromatic ring will enhance the anion’s stability around the negatively charged atom because of resonance.
A point to be noted: the bigger the resonance structure, the more stable it will be.
Due to resonance, the negative charge on benzyl carbanion disperses over additional carbon atoms, making it more stable than ethyl carbanion.
Stability of free radicals
Due to depolarisation of the unpaired electrons across the system, simple alkyl radicals are less stable allylic and benzylic forms of free radicals.
Mesomeric effect vs resonance effect
- Resonance effect can be defined as the process in which two or more structures can be written for the real structure of a molecule, but none of them fully explains all characteristics of molecules. Substituents or functional groups in a chemical molecule cause the mesomeric effect, denoted by the letter M.
- Delocalisation of electrons in a system is known as resonance whereas the mesomeric effect is known as the resonance effect. It is a long-term impact that is reliable on the substituents or functional groups in a chemical compound.
- The +R (electron releasing) group is equal to the +M effect, while the –R (electron attracting) group is equal to the –M effect.
Principle of resonance
- The most fundamental resonance is the one that is generated with the least charge.
- The resonance of a full octet is more substantial than that of a partial octet. The most essential forms are those in which positive charges operate on the least electronegative atom.
- The resonance structure with the greatest covalent bond is the most significant.
Resonance effect vs inductive effect
- An inductive effect occurs when the polarisation of one link is caused by another link. On the other hand, the resonance effect occurs when two or more structures may be described for molecules, but none can describe all the characteristics of a molecule on their own.
- The difference in electronegativity between two atoms in a bond affects the inductive effect directly, whereas the number of resonant structures affects the stability.
Occurrence of resonance
- A pi bond conjugated with the other pi bond
- A pi bond conjugated with a negative charge
- A pi bond with a positive charge conjugated to it
- A negative charge conjugated with the lone pair or a positive charge conjugated with a lone pair
- A pi bond conjugated with a lone pair or a free radical
Conclusion
In chemistry, resonance is an intramolecular electrical phenomenon in which the location of a pi bond(s) or a nonbonding electron changes (also called a sigma bond). In this procedure, however, the location of an atom is changed by modifying the pi electrons’ position or the non-bonding electrons’ position.
Resonance is a property of organic compounds. In organic chemistry, the delocalised electrons inside a specific compound when a single Lewis structure does not express the bond are referred to as resonance. To portray delocalised electrons in an ion or molecule, several structures known as resonance can be used.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out