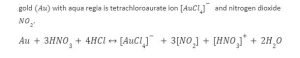Introduction
Depending on the position of metals in the reactivity series, metals react when they come in contact with water, acid or oxygen. The reactivity series contains elements of higher reactivity at the top and lower reactivity at the bottom. Gold, being one of the noblest elements, lies at the bottom of the series..
Reaction of Metals with Water
When any metal reacts with water, the product formed is hydrogen and metal hydroxide. The reaction with metals and water is summed as:
Metal + Water Hydrogen + Metal hydroxide
Some metals react faster than others. Metals like potassium, sodium and lithium are so vigorous and violent that they need to be stored in oil. They can even act with the moisture in the air.
Water Reaction with different Metals:
- Reaction of Sodium with Water
When sodium reacts with water, a lot of heat is produced, and the mixture catches fire. Sodium is very vigorous and highly reactive.
The chemical equation for the reaction of sodium with water is given as

Reaction With Metals with Acid
When a metal reacts with the acid, the product formed is hydrogen and metal salt.
The general reaction with metals and acid is summed as Metal+Acid —–Hydrogen+Metal salt
The nature of the salt depends on the metal reacted, and the acid used. Metals like copper, silver, gold and platinum are inert to acids.
Acid Reaction with different Metals:
- Reaction of Aluminium with Acid
When aluminium reacts with dilute hydrochloric acid, the reaction produces aluminium chloride and hydrogen gas. It is an irreversible reaction. The reaction is a kind of redox reaction.
The chemical equation for the reaction of aluminium with hydrochloric acid is given as
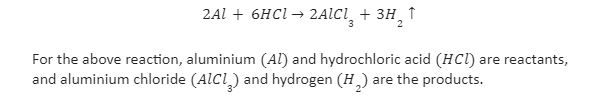
- Reaction of Magnesium with Acid
When magnesium reacts with hydrochloric acid, the reaction produces magnesium chloride and hydrogen gas. Magnesium acts as a reducing agent in the chemical reaction, and hydrochloric acid acts as an oxidising agent.
The chemical equation for the reaction of magnesium with hydrochloric acid is given as
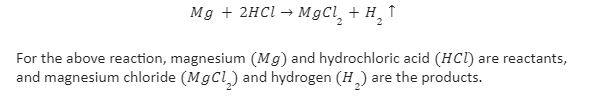
Reaction With Different Metals and Oxygen
Whenever a metal reacts with oxygen, the metal oxide is produced. Sometimes metals need to be burnt to make them react with oxygen. The process that involves burning is known as the combustion process.
Any reaction with metals and oxygen is summed as
Metal+OxygenMetal Oxide
Oxygen Reaction with Different Metals:
- Reaction of Iron with Oxygen
When kept in the open air, the metal iron reacts with the oxygen present in the air and forms rust. This rust is chemically called iron oxide.
The chemical equation for the reaction of iron with oxygen is given as
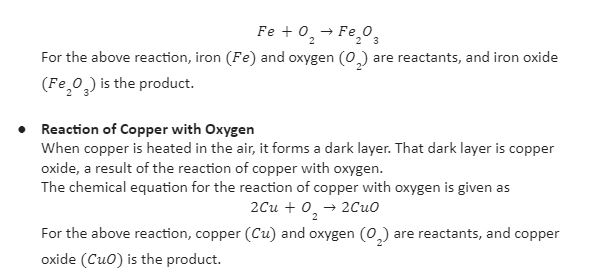
Conclusion
Metals present at the top of the reactivity series like potassium, sodium, lithium, barium and strontium are highly reactive. They can react with water, acids and oxygen. The middle part of the reactivity series contains magnesium, manganese, aluminium, chromium, cadmium, iron, cobalt, nickel, zinc, tin and lead. These are metals with moderate reactivity. They can react with acids and oxygen but not water. The last part of the series contains elements that are significantly less reactive. It includes copper, mercury and silver. They only react with oxygen.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out