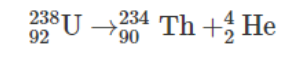Radioactivity is the process by which the molecule emits unstable atoms from the nucleus to maintain a stable configuration. It is vital because the molecule could acquire more atoms to increase its size as time passes, making it highly concentrated and packed with a cluster of energy. This clustered energy could make the molecule extremely unstable. This is why atoms of Plutonium, Uranium are extremely rare and expensive, as they are unstable and undergo the phenomenon of radioactivity.
Carbon dating is a form of radioactivity and is carried out by the decaying properties of Carbon-14 isotopes. This process is mainly adapted by architects for determining the age of a material. According to physics, the decaying rate of the material is proportional to the mass and the atomic number of the decaying atom. With this property, we have the advantage of finding out the approximate age of the decaying material by taking the ratio of the concentration of isotope in the present decaying material to the concentration of isotope during the alive lifespan. To have a more detailed grip on this topic, continue reading radioactivity and carbon dating study material.
Importance of Carbon Dating
Carbon dating allows mankind to learn about human civilization, religion, and culture from earlier times. It helps researchers understand and research the climatic and environmental changes compared with the prehistoric period. Without the occurrence of Carbon dating, human generations couldn’t have upgraded themselves from the concept of hypothesis and assumptions as they have learned so many things from their bygone ancestors. Also, for studying the planet’s history when it was born, it is important to adapt to carbon-14 dating since it has maintained its position as the primary tool for dating material with a lifespan of 50,000 years.
What are the Types of Radioactive decay?
Radioactive decay is the property of emitting atoms from a chemical compound to withstand a stable electronic configuration. The atoms are radiation emitted by the unstable atom’s nucleus. The elements that undergo emitting radiation and radioactive decay are called radioactive elements. These elements cannot bind more atoms to it, as there is no energy left due to an unstable nucleus. However, they can become stable by adapting to the process of transmutation.
Transmutation is the process of transforming an isotope into a stable nucleus. This process can be achieved either naturally or artificially. For transmutation, radioactive decay is classified into three types: Alpha Decay, Beta Decay, and Gamma Decay. Proceed with our study material notes on radioactivity and carbon dating for better understanding.
- Alpha Decay: This type of decay occurs when a high-energy helium nucleus is emitted. The formula for alpha decay is given as:
![]()
Mi is the initial mass of the nucleus.
MF is the mass of the nucleus after the emission of particles
Mp is the mass of the emitted particle.
- Beta-decay: A beta decay happens when the emitted atom is an electron, but it could be a position too. An example of beta decay is shown below:
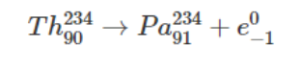
- Gamma decay: While rotating in orbit, the electron possesses some energy, and when an electron jumps to change its orbit from a higher energy level to a lower, the difference makes a photon to be emitted. The same process happens with the nucleus; when the nucleus arranges for a lower energy level, a high-energy photon is shot out, known as a gamma-ray.
What are the Laws and Uses of Radioactivity?
A scientist named Henry Becquerel discovered the property of radioactivity through an accident. He placed a Uranium compound inside a closed packed drawer containing photographic plates and wrapped it in black paper. After some time, when the drawer was examined, traces of radioactivity were seen. To study it in detail, he imposed some laws necessary for understanding the property of radioactivity.
Laws
- The decaying rate of the radioactive substance depends on the number of atoms present at the time.
- The emission of energy is always accompanied by alpha, beta, and gamma rays.
- The chemical and physical properties are dissimilar between the mother and daughter nuclei.
- The property of radioactivity is proportional to the property of the law of conservation of charge.
- The decaying rate of the substance does not get affected by the temperature and pressure surrounding it.
- The process of radioactivity is the result of the decay of the nucleus.
Uses of Radioactivity
- Gamma rays are used in treating cancer-affected cells and hence used in radiotherapy.
- To destroy carcinogenic cells, Cobalt-60 is used.
- Gamma rays are used to scan the internal parts of the body.
- Gamma rays can also kill microbes present in food and prevent it from decaying.
- The ancient age can be studied through carbon dating or by measuring the argon content present in the rock.
- Radioactivity can be used in domestic smoke detectors and medical instruments.
- It can also be used to sterilise medical instruments and produce electric power.
What is an Alpha Decay?
Alpha decay happens in a molecule when the unstable nucleus emits a helium nucleus, i.e., an alpha particle. The ejected particle contains four nucleons which include two neutrons and two protons. An alpha decay makes the molecule achieve a more stable electronic configuration by reducing the ratio of protons to neutrons in the parent nucleus. The alpha particle comprises two protons and neutrons while exiting the nucleus, changing the atomic number for the element left behind. The element left behind replaces the atomic number with a value that is two digits lesser and a mass number that is four digits less. For better understanding, let’s consider an example of Uranium decaying to form Thorium.
Conclusion
The concept of radioactivity and carbon dating is very crucial to learn. Radioactivity is the emission of electrons to maintain a steady-state, while carbon dating is used for historical purposes. Without the property of radioactivity, unstable molecules tend to be growing weakly. Also, it plays a vital role in many fields like medical, educational, and industrial. People can also use the property of radioactivity for generating electricity. This radioactivity and carbon dating study material provided above could help you understand the topic more quickly and efficiently. Studying all topics from the radioactivity and carbon dating study material can help you achieve the milestone over the concept of radiation in chemistry.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out