Fructose refers to the simplest form of sugar-like monosaccharide found in various products. It is considered one of the important blood sugars, including glucose and galactose. Fructose is found in honey, tree fruits, berries, melons, and some root vegetables such as sweet potatoes and beets, and others, frequently in conjunction with glucose and sucrose.
Fructose is produced through sucrose digestion, a disaccharide composed of fructose and glucose. This is further broken down during the digestion process through glycoside hydrolase enzymes.
This article will cover various fructose questions with the physical properties of fructose, structure, and fructose examples.
Fructose
Fructose is a simple “ketonic monosaccharide sugar” found in several plants, flowers, and fruits. It is a fruit sugar that, together with glucose and galactose, is one of three dietary monosaccharides directly absorbed into the blood during digestion.
Monosaccharides are the most basic unit of carbs and the typical example of sugar. As a result, fructose is the most basic sugar and is more easily digestible than other sugars. It is a pleasant, white, odourless, crystalline solid in its purest form. It is more water-soluble than other sugars.
It is commonly found in many foods such as litchi, cherry mango, guava and vegetables such as carrot, beetroot, radish, and sugarcane. It is derived commercially from maize, sugar cane, and sugar beets.
Fructose, like all monosaccharides, is a lowering sugar. Glycation, or the random attachment of single sugar molecules to proteins, is a significant cause of damage in diabetics.
Fructose appears to be just as harmful as glucose in this aspect and hence does not appear to be the solution for diabetes. This could play a significant role in ageing and many age-related chronic diseases.
Physical properties of fructose
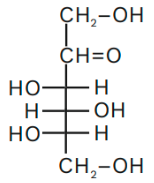
Fructose crystals have a ring configuration. It is a polyhydroxy ketone with six carbons. “Hemiketal and internal hydrogen bonding” help it to be strong. In this form, it is known as D – fructopyranose. In its aqueous solution, fructose exists as an equilibrium combination of 70% fructopyranose, 22% fructofuranans, and 7% three additional forms, such as its acyclic structures. Six carbon atoms, twelve hydrogen atoms, and six oxygen atoms are fructose. At carbon number 2, this has a ketone functional group. As a result, this is a ketohexose. It is obtained in conjunction with glucose.
The basic properties of fructose are:
- It has a molecular mass of 180.156 g.mol-1.
- It has a melting point of 103 °C.
- This is the most water-soluble sugar.
- It has a sweet flavour. It is employed as a natural sweetener in beverages and meals. It is the cheapest and tastiest naturally occurring carbohydrate. Its relative sweetness reduces as the temperature rises.
- At standard temperature, this is a white crystalline solid.
- It’s a flavourless sugar.
- It possesses a high hygroscopicity, or the capacity to receive moisture from its surroundings. Compared to other sugars, it absorbs moisture quickly and releases it slowly.
- It is a good humectant, which means it can hold moisture. It can also keep the moisture that has been absorbed.
- Yeast and bacteria can ferment fructose anaerobically. Yeast breaks down sugar structure into carbon dioxide and ethanol.
- It demonstrates the Maillard reaction. Since it can remain in open-chain form for a more extended period, the early stage of the ‘Maillard reaction’ happens more quickly.
- It can produce mutagenic chemicals.
- Fructose, when dehydrated, yields hydroxymethylfurfural.
This is the formulation and structure of fructose.
Importance of fructose
Fructose is frequently suggested and ingested by patients with diabetes or hypoglycemia as it has a very glycemic index compared to cane sugar or sucrose. However, there is some worry that fructose may also have a negative impact on uric acid levels and plasma lipids, and that increased fructose levels in the blood may be harmful to proteins. The low GI is owing to a complex metabolism pathway and fructose distinct, which includes phosphorylation and a multistep enzymatic activity in the liver.
Examples of fructose
Fructose is classified into two types: crystalline fructose and high-fructose corn syrup (HFCS).
Crystalline fructose: It is entirely made up of fructose components of 100% fructose. It is widely regarded as among the best pure sweeteners. Cakes and other baked goods benefit from the addition of crystalline fructose, which enhances the texture and consistency. It also gives the eatables a visually beautiful brown colour.
High-fructose corn syrup: In general, sucrose and HFCS contain the same quantity of glucose and fructose. The element ratio of HFCS is typically 55% for fructose and 45% for glucose.
Conclusion
Fructose is cost-effective while having no influence on measured blood glucose. It is often used to replace sucrose or ordinary sugar. Fructose is frequently consumed in the form of HFCS. The corn syrup is composed of glucose that has been enzymatically treated by the ‘enzyme glucose’ to transform a part of the glucose into fructose, increasing its sweetness.
However, fructose has been linked to obesity, increased LDL cholesterol and triglycerides, and metabolic syndrome. In contrast to animal studies, some human studies have failed to demonstrate a link between fructose consumption and obesity.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





