In studying organic chemistry and organic compounds, amines play a crucial role. This is because amines are derivatives of ammonia, in which one or more than one hydrogen is simply substituted with an alkyl group or aryl group. The basis of classification of amines into primary, secondary and tertiary, etc., is done based on how many hydrogens have been substituted by the alkyl or aryl group. Examples of primary amines are
Propylamine CH3CH2CH2NH2, allylamine etc.
Primary amines are ammonia derivatives with only one hydrogen atom substituted by alkyl or aryl. In simple words, it can be termed as the amines containing only one alkyl or aryl group attached to the ammonio group. Thus, only one of the hydrogen is being substituted. This amine type has significant intermolecular interaction, leading to more strong bonding.
The reason for this strong intermolecular interaction in primary amines is that the number of hydrogens is more than other types of amines, that is, secondary, tertiary and quaternary amines. Thus, the primary amines are the only type of amines that are liquids, except this all are solid at room temperature.
Nomenclature of primary amines
To have uniformity of names of compounds, the International Union of Pure and Applied Chemistry, IUPAC, came up with a universal pattern for naming organic compounds.
Thus, all the organic compounds are named as per IUPAC nomenclature norms, and the amines are also named similarly and the letter “e” is replaced by the word “amine” as IUPAC nomenclature rules do not allow two vowels together.
The chain containing the amino group is considered the primary chain. The groups attached to nitrogen atoms are located using “N” as the position number.
However, apart from IUPAC nomenclature, some common names are used widely. For example, in common nomenclature, the primary amines are termed alkylamines.
Physical properties of primary amines
Below stated are the properties of primary amines:
Water solubility: The water solubility of amines depends on several factors like hydrophobicity, polarity, bond strength, intermolecular hydrogen bonding, etc. The primary amines have a lesser hydrophobic alkyl group, leading to the decreased molar mass of amines.
Primary amines can form hydrogen bonds with water. All these factors result in higher water solubility than secondary and tertiary amines because the number of hydrogen is lesser, and the number of the alkyl group is more in secondary amines followed by tertiary amines.
Boiling point: Primary amines have the highest boiling point due to maximum intermolecular strength and intermolecular hydrogen bonding. The number of hydrogens is more in primary amines than others.
Colour: Primary amines (lower primary amines ) specifically are mostly colourless. However, they can acquire colour due to oxidation if left open and exposed to air.
Chemical properties of primary amines
Basicity: Amines, when treated with an acid, forms salt, which simply suggests that it is basic.
The primary amine is least basic because the presence of an electron-donating group increases the basicity of the amines, the electron-donating group is the group that release electrons, and in amines, it includes alkyl groups; thus, lesser the number of alkyl groups attached to the amine group lesser is the basicity.
Thus, primary amine is least basic, followed by secondary, tertiary and quaternary amines as they have more electron-donating groups.
Reactions of primary amines:
Gabriel synthesis: it is one of the most important reactions of organic chemistry and one of the most effective ways to synthesise the primary amine as there is no change in the number of carbon atoms.
In this reaction, phthalimide is reacted with ethanolic potassium hydroxide leading to the formation of the potassium salt of phthalimide which is further heated with an alkyl halide leading to the formation of alkyl phthalimide.
The product formed after treatment with an alkyl halide, alkyl phthalimide, leads to the formation of primary amine by undergoing alkaline hydrolysis.
This method can only synthesise primary amine and not secondary and tertiary because over-alkylation is avoided.
Gabriel Phthalimide Reaction
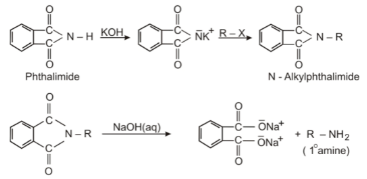
Aromatic primary amines can also not be prepared because aryl halides do not undergo nucleophilic substitution with potassium salt or phthalimide.
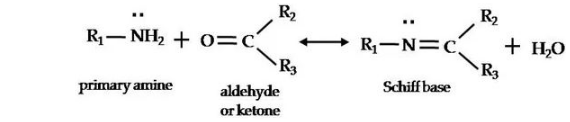
- Schiff base is formed when the primary amine is reacted with aldehyde and ketone. This is a dehydration reaction.
- The test to distinguish between primary, secondary and tertiary amines is known as the Ginsberg test; this test is based on the formation of sulfonamides. in this test, benzene sulfonyl chloride is reacted with an amine if the product is formed then either it is a primary amine or secondary amine but not tertiary amine. Thus, it rules out the possibility of a tertiary amine. now, to distinguish between primary and secondary, it is checked whether the product formed dissolves in aqueous sodium hydroxide or not. If it dissolves in it, it is primary amine; if not, it is a secondary amine.
Conclusion
Primary amines have only one alkyl group and have the least basicity. Due to lesser molecular mass and higher intermolecular strength, and hydrogen bond strength, it is the most soluble among all the amines. It has the highest boiling point among amines. It can be synthesised using Gabriel synthesis and distinguished using the Hinsberg test.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





