A colloid is another name for a colloidal solution. It is a heterogeneous solution, but due to the small particle size, it may appear to be a real solution. To distinguish a colloid from a genuine solution, the term ‘sol’, as in colloidal sol, is used instead of the term ‘solution’. Their particles are also referred to as colloidal particles.
Completely dissolving particles provide an osmotic force that attracts water in a solution.
A colloid is a silent solution with solute particle sizes that are halfway between those in real solutions and those in suspensions, for example, milk and water.
The solute particles in a real solution are so tiny that they cannot scatter or reflect light rays that fall on them.
Preparation of Colloid Solutions
Colloid solutions can be prepared using the following methods:
- Chemical method: Colloidal solutions are frequently made by a reaction that produces molecules. Sols are formed when these molecules assemble.
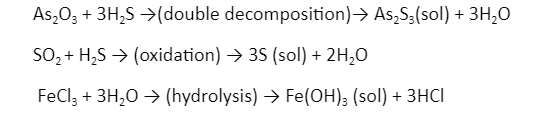
- Electrical disintegration: Dispersion works effectively as condensation in this case. Sol is used to make gold and silver metals. This procedure involves striking a discharge between metal electrodes immersed in the dispersing phase. The extreme heat causes the metal to vaporise, which then condenses into colloidal particles.
- Peptization: The addition of some electrolytes in the dispersed phase and vigorously mixing it turns the ppt into a colloidal sol. The electrolyte is used as a peptizing agent in this case. A colloidal solution can also be formed when a precipitate is shaken; this process is referred to as peptization. This results in a +ve charge on the precipitate, which eventually breaks into little particles with colloid-like dimensions.
Tyndall Effect
The Tyndall effect refers to the scattering of sunlight by colloidal particles. The Tyndall effect occurs when a fine beam of sunlight enters a region through a small hole and is scattered by dust and smoke particles in the air. For example, a solution of copper sulphate does not have a Tyndall effect, but a mixture of water and milk does.
When sunlight penetrates through a dense forest canopy, Tyndall effects are common. Mist in the forest contains microscopic droplets of water that behave as colloid particles distributed throughout the air.
A true solution differs from a sol in that a true solution does not scatter a beam of sunlight travelling through it, whereas a sol scatters a beam of sunlight passing through it and makes its path apparent.
Colloids are varied in nature, despite their appearance.
Properties of Colloid Solutions
- A colloid is heterogeneous; it pretends to be homogenous.
- The size of the particles during a colloid is greater than those during a true solution but smaller than those during a suspension. It’s between 1 nanometre and 100 nanometre in diameter.
- The particles of most of the colloids can’t be seen even with a microscope.
- It scatters a beam of sunshine passing through it.
- There is a special technique referred to as centrifugation that separates the colloidal particles from a sol.
- The components of soil are the dispersed particles and, therefore, the dispersing phase.
- The solute-like component or the dispersed particles during a colloid form the dispersed phase, and therefore, the component during which the dispersed particles is suspended is understood because of the dispersing phase.
- Colloids are classified as consistent with the state (solid, liquid, or gas) of the dispersing phase and, therefore, the dispersed particles.
Classification of Colloid Solutions
- Sol refers to a colloidal dispersion of solid particles in a liquid.
- The mixture of two liquids is called emulsion.
- Foam is formed when a significant number of gas particles become trapped in liquid or solid.
- Aerosols are gaseous suspensions of tiny liquid or solid particles.
The colloidal system is called a hydrocolloid when the dispersion medium is water. The particles in the dispersed phase may move through multiple stages depending on how much water is available. When Jell-O powder is mixed with water, it forms a hydrocolloid. Hydrocolloids are commonly used in medical dressings.
Conclusion
A colloidal solution is a mixture in which the solute particles are suspended throughout the solution and have a particle size that is intermediate between that of actual solutions and suspensions. Colloidal solutions contain particles that are larger than those found in a true solution but smaller than those found in suspensions. Particles smaller than 1 nanometer, colloids between 1 and 1000 nanometers, and suspensions greater than 1000 nanometers are examples of true solutions. Another differentiating feature that distinguishes colloidal solution from suspension is that the particles do not settle at the bottom when left undisturbed.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






