There are various factors that affect the rate of a reaction. These factors include chemical factors of reactants like the nature of bonding, thermodynamics, kinetics, and physical factors like the physical state of reactants in which they exist, temperature, pressure, concentration, presence or absence of a catalyst, and so on. In order to undergo a balanced reaction, both the chemical factors of reactants as well as the physical state of reactants need equal consideration.
For a reaction to occur, it is very important for the reactants to come in contact with each other. If the reactants are in different phases, they react with each other only in the interface. For a complete reaction to occur, the nature of the participating reactants needs to be known.
It’s important to mention the physical state of reactants when denoting a chemical reaction. This ensures the reader understands the phases of reactants, the applied pressure and temperature, the role of the catalyst in the reaction, and other basic parameters. In most of the reactions, common notations like (s) for solids, (l) for liquids, (g) for gases and (aq) for aqueous medium are mentioned. These denote the phases of the reaction. Apart from this, the temperature, pressure and catalyst information is mentioned on the arrow that directs towards the product. If the reaction is reversible and can proceed in both directions, then two half arrowheads (⇌) are used, whereas if the reaction is irreversible and proceeds only in one direction, i.e., forward direction, then a single arrow (→) directing towards the product is used.
Notation Used to Describe the Physical State of Reactants
Symbol/Notation | Meaning |
s | Solid |
l | Liquid |
g | Gas |
aq | Aqueous |
ppt | Precipitate |
Δ | Heat |
T | Temperature in degree/Kelvin |
| ↑ | Heat/gases are evolved in the system |
Physical Factors Affecting Reactions
Temperature of the reactants
Reactions usually occur faster when the temperature of the reaction is increased. We might have used ovens, burners, and hot plates in the laboratory to speed up the reaction. On the other hand, the shelf life of food kept in the refrigerator increases as the process of decaying becomes slower. The rate of the reaction is doubled when the reaction temperature is increased.
Concentration of the reactants
Concentration of a reaction is directly proportional to the rate of the reaction. If the reactant concentration is doubled, the reaction will occur faster. For instance, calcium carbonate deteriorates rapidly in the presence of sulphur dioxide. As the concentration of sulphur dioxide in the air increases, the amount of calcium carbonate undergoing deterioration doubles. Consider the following reaction:
The sulphurous acid used in this reaction is from the atmospheric sulphur dioxide that reacts with moisture to form sulphurous acid.
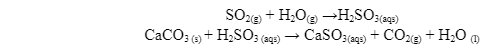
Catalyst
Any substance that increases the rate of reaction or lowers the temperature or pressure required in the reaction without itself being consumed in the reaction is called a catalyst. The presence of a catalyst plays an important role in speeding up the reaction. Consider the following reaction:
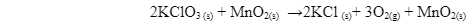
In the above reaction, the reduction of potassium chlorate to potassium chloride is faster due to the presence of manganese oxide. If we observe the reaction, the catalyst is retained at the end of the reaction, indicating it’s a positive catalyst.
Surface area of the reactant
Surface area is another important parameter that helps in a faster reaction. It is directly proportional to the speed of the reaction. For example, if solid and gas reactants are mixed with each other, only the surface molecules of the solid will interact with the gas molecules and not the molecules present in the core. But if the same solid is spread in a flat surface, more molecules will have an interaction with the gasses.
Conclusion
While there are various factors that are considered for a reaction to occur, the physical state of reactants plays an important role in determining the rate at which the reaction proceeds. Some of these factors like temperature, catalyst, concentration and surface area, with relevant examples, have been discussed in this section.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





