The number of electrons that each element gains or loses in order to complete its outermost shell is referred to as its valency. When an atom’s outer shell, or octet, is completed, it becomes more stable (8 electrons ions in the outermost shell). As a result, element valence or oxidation states interact with other atoms or participate in chemical interactions in which they can donate or lose electrons to other atoms, or take or acquire electrons from other atoms to produce a stable state.
What are valence electrons?
The total number of electrons in an atom’s outermost orbit, or energy shell, is known as valence electrons. Magnesium, for example, has an electrical structure of 2, 8, 2. The K orbit has two electrons, the L orbit has eight, and the M orbit has two. Magnesium has two electrons in its outermost orbit. It has two valence electrons as a result.
What is valency?
Valence/valency is a property of an element that impacts how many other atoms it can mix with in chemistry. The combining capacity or affinity of an atom of a certain element is determined by the number of hydrogen atoms it can combine.
What is the condition of oxidation of elements?
The oxidation state refers to the maximum amount of electrons that an atom can lose or gain in order to form a bond with another atom. Diatomic molecules are valency 1 diatomic elements.
Periodicity of valence or oxidation states:
Oxidation State and valency are two of the most fundamental features of components that may be investigated using electron configuration or the number of electrons in the valence shell of an atom (outer shell).The valencies of elements in the s-block and p-block of the periodic table are calculated using the number of valence electrons or eight minus the number of valence electrons.
For the d-block and f-block elements, valency is determined not only by valence electrons but also by d and f orbital electrons. The generic valencies of these d-block and f-block components, on the other hand, are 2 and 3.Valence electrons are electrons that are found in a molecule’s outermost shell.At the same time, the number of valences determined an atom’s valency or valence.
Types of valency:
Electrovalence:
The property of atoms that generate ionic or electrovalent compounds is called electrovalence. Electrovalence is the number of electrons lost or gained by an atom in order to achieve a stable state or complete its octet. When an atom loses electrons, positive ions (cations) develop, and their valency is known as positive electrovalence. When atoms receive electrons, negative ions (anions) develop, and their valency is known as negative electrovalence. So, when metals and non-metals come together, electrovalence refers to the valency of ionic (electrovalent) compounds or electrovalent compounds. Sodium chloride (NaCl), for example, is a non-metal containing a metal component.
The chemical bond between the sodium and chlorine atoms in the Sodium Chloride molecule is formed by the transfer of electrons from Sodium to Chlorine. One electron is gained by chlorine, while one is lost by sodium. The electrovalence of sodium and chlorine is 1 because Na loses one electron and Cl receives one, as shown below.
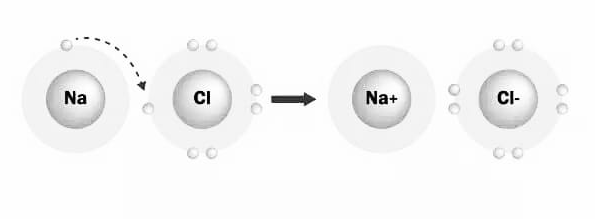
Covalency:
During the creation of a covalent molecule, the number of electrons shared by atoms or elements is referred to as the number of electrons shared by the atoms or elements. For periodicity of valence or oxidation states of elements, the chemical link between non-metals is established in covalent compounds. When atoms of one non-metal combine with atoms of other non-metals to form molecules, covalent compounds are created.In this arrangement, the atoms do neither gain or lose electrons; instead, they share electrons. One carbon atom, which requires four electrons to complete its octet, joins four hydrogen atoms, each of which requires one electron to complete the two electrons required in its initial orbit in Methane (CH4).
Conclusion:
The valencies of each of an atom’s 118 elements refer to how many electrons it acquires or loses to complete its outermost shell. Atoms become more stable as their outer shell, or octet, is completed (8 electrons in the outermost shell). As a result, periodicity of valence or oxidation states of elements interacts with other atoms or participates in chemical interactions in which they can give or lose electrons to other atoms, or take or acquire electrons from other atoms, resulting in the development of a stable state. The number of electrons present in the outermost shell is the same when evaluating the electron configurations of elements in the same group in the periodic table.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





